What Are Immunodeficiency Disorders?
Immunodeficiency disorders are those which impair the immune system’s ability to defend the body against foreign or abnormal cells that invade or attack it such as bacteria, viruses, fungi, and cancer cells.
Immunodeficiency disorders include primary immunodeficiencies, defects in innate immunity, defects in adaptive immunity, defects in lymphocyte maturation, defects in lymphocyte activation and function, immunodeficiencies associated with systemic diseases, secondary immunodeficiencies, amyloidosis, and acquired immunodeficiency syndrome (AIDS).
What is the Normal Immune Response?
The normal immune response is way the body defends itself against substances it sees as harmful or foreign. The immune system recognizes the antigens (usually proteins) on the surface of substances or microorganisms, and attacks and destroys, or tries to destroy, them.
Immunity is the ability of an organism to resist a particular infection or toxin by the action of specific antibodies or sensitized white blood cells.
What Is Innate Immunity?
Innate immunity is Immunity that is naturally present and is not due to prior sensitization to an antigen from, for example, an infection or vaccination. Since it is not stimulated by specific antigens, innate immunity is generally nonspecific.
The components of innate immunity: anatomic barriers (skin and mucous), physiologic barriers (temperature, pH, and chemical mediators), phagocytic barriers (specialized cells), and inflammatory barriers (leakage of vascular fluids).
The major cells of the immune system are:
- Dendritic cells
- Epithelial cells
- Mast cells
- Monocytes
- Natural killer cells
- Neutrophils
What Are the Receptors for Microbes, Damaged Cells, and Foreign Substances?
Pathogen-associated molecular patterns Pathogen associated molecular patterns (PAMP) are molecules with conserved motifs that are associated with pathogen infection that serve as ligands for host pattern recognition molecules such as peptidoglycan, lipoteichoic acids and mycolic acid.
Damage-associated molecular patterns are molecules within cells that are a component of the innate immune response released from damaged or dying cells due to trauma or an infection by a pathogen.
Toll-like receptors a class of pattern recognition receptors (PRRs) that initiate the innate immune response by sensing conserved molecular patterns for early immune recognition of a pathogen
NOD-Like receptors (a subset of pattern recognition receptors) are a specialized group of intracellular proteins that play a critical role in the regulation of the host innate immune response.
Inflammasomes are innate immune system receptors and sensors that regulate the activation of caspase-1 and induce inflammation in response to infectious microbes and molecules derived from host proteins.
C-type lectin receptors (CLRs) are a large family of transmembrane and soluble receptors that contain one or more carbohydrate-recognition domain able to recognize a wide variety of glycans on pathogens or on self-proteins.
Retinoic acid-inducible (RIG) like receptors are a type of intracellular pattern recognition receptor involved in the recognition of viruses by the innate immune system.
What are the Reactions of Innate Immunity?
Reactions of innate immunity are inflammation, antiviral defines, signal generation.
Inflammation is a process by which your body’s white blood cells and the things they make protect you from infection from outside invaders, such as bacteria and viruses.
Antiviral defines: Protein synthesized or activated in the cell in response to viral infection, or protein with specific antiviral activity within the cell.
Signal generation is done by molecules released by stressed cells undergoing necrosis that act as endogenous danger signals to promote and exacerbate the immune and inflammatory response.
What is Adaptive Immunity?
Adaptive immunity involves specialized immune cells and antibodies that attack and destroy foreign invaders and are able to prevent disease in the future by remembering what those substances look like and mounting a new immune response.
There are two types of adaptive immunity are cell mediated immunity and humoral immunity.
Cell mediated immunity is an immune response that does not involve antibodies. Rather, cell-mediated immunity is the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen.
Humoral immunity is also called antibody-mediated immunity. With assistance from helper T cells, B cells will differentiate into plasma B cells that can produce antibodies against a specific antigen.
Cells of the immune system are lymphocytes (T-cells, B-cells and NK cells), neutrophils, and monocytes/macrophages.
T lymphocytes are major components of the adaptive immune system. Their roles include directly killing infected host cells, activating other immune cells, producing cytokines and regulating the immune response.
Helper T cells also called CD4+ cell type of white blood cell that serves as a key mediator of immune function which play a central role in normal immune responses by producing factors that activate virtually all the other immune system cells.
Cytotoxic T cells are effector cells that destroy virus-infected cells, tumour cells, and tissue grafts that exist in the cytosol, or contiguous nuclear compartment. The cells are also known as CD8+ T cells as they express the CD8 glycoprotein at their surfaces.
Natural killer cells Natural killer cells are a type of lymphocyte (a white blood cell) and a component of innate immune system.
These cells play a major role in the host-rejection of both tumours and virally infected cells. They usually express the surface markers CD16 and CD57 in humans.
B lymphocytes are the effectors of humoral immunity, providing defines against pathogens through different functions including antibody production. Immature B cells express CD19, CD 20, CD34, CD38, and CD45R.
Dendritic cells are immune cells that link innate and adaptive immunity. The main function of these innate cells is to capture, process, and present antigens immune cells. Some markers for dendritic cells are CD1A and CD14.
Macrophages are specialised cells involved in the detection and phagocytosis. Markers for CD antigens include CD16 and CD14.
Generative (primary) lymphoid organs
1. Bone marrow is a spongy substance found in the centre of the bones. It manufactures bone marrow stem cells and other substances, which in turn produce blood cells
2. The thymus is a specialized primary lymphoid organ of the immune system. Within the thymus, thymus cell lymphocytes or T cells mature.
Peripheral (secondary) lymphoid organs
1. Lymph nodes small bean-shaped structure that are part of the body’s immune system which filter substances that travel through the lymphatic fluid
2. The spleen is a small organ inside left rib cage, part of the lymphatic system. The spleen stores and filters blood.
What Are Major Histocompatibility Complexes (MHCs)?
Major histocompatibility complexes (MHCs) are a group of genes that encode proteins on the cell surface that have an important role in immune response. Their main role is in antigen presentation.
Class I MHC molecules are cell surface recognition elements expressed on virtually all somatic cells. Each human cell expresses six MHC class I alleles.
Class II MHC molecules normally found only on professional antigen-presenting cells such as dendritic cells, mononuclear phagocytes, some endothelial cells, thymic epithelial cells, and B cells. There are six to eight MHC class II alleles.
MHC locus is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system.
What Are Cytokines?
Cytokines are small proteins that are crucial in controlling the growth and activity of other immune system cells and blood cells.
| No. | Cytokines | Source | Target | Biological role |
| 1 | IL-1(IL-1α and -β) | Macrophages, dendritic cells, endothelial cells | TH and B cells and various other tissues | Activation |
| 2 | IL-2 | TH1 cells | TH, Tc and NK cells | T cell and NK proliferation and induction of activity |
| 3 | IL-3 | TH, Tc and NK cells | Hematopoietic and mast cells | Progenitor cell proliferation and differentiation |
| 4 | IL-4 | TH2 cells, mast cells, NK cells | B cells, T cells, mast cells, macrophages | Proliferation, isotype switching, induction of MHC class II expression |
| 5 | IL-5 | TH2 cells, mast cells | Eosinophils | Proliferation and differentiation |
| 6 | IL-6 | Macrophages, TH2 cells | Plasma cells, B cells and others | Differentiation and antibody secretion |
| 7 | IL-8 | Bone marrow, thymus | Neutrophils | Chemoattractant |
| 8 | IL-9 | TH2 cells | TH cells, mast cells, eosinophils | Induces inflammatory responses |
| 9 | IL-10 | TH2 cells | Macrophages, APC | Anti-inflammatory cytokine inhibits cytokine production |
| 10 | IL-11 | Bone marrow | B-cell progenitors and others | Differentiation |
| 11 | IL-12 | Macrophages, B cells | Tc, NK and LAK cells | Proliferation and differentiation in synergy with IL-2 |
| 12 | IL-13 | TH cells | Macrophages, B cells | Inhibition of inflammatory cytokines, regulation of inflammation. Parasitic infections |
| 13 | IL-16 | Tc cells | TH cells | Chemotaxis |
| 14 | 1L-18 | Hematopoietic and nonhematopoietic lineage cells | T cells, NK cells | Proinflammatory cytokine; IFN-γ inducing factor |
| 15 | IFN-α | Leukocytes | Inhibitor of viral replication | |
| 16 | IFN-β | Fibroblasts | Inhibitor of viral replication | |
| 17 | IFN-γ | TH1, TC, NK | Various cells including macrophages | Inhibitor of viral replication, Inhibitor of cell proliferation. Inhibitor of IL-4 induced isotype switching |
| 18 | TNF-α | Macrophages | Tumour cells, polymorphonuclear leukocytes, macrophages | Cytotoxicity, induction of cytokine secretion |
| 19 | TNF-β | T cells | Tumour cells, neutrophils, macrophages | Cytotoxicity, phagocytosis |
How Are Lymphocytes Activated?
Lymphocytes are activated through antigen-specific receptors on their cell surface. This causes the cells to proliferate and differentiate into specialized effector lymphocytes. Display of antigens allows T cells to recognize antigenic epitopes presented on the surface of an antigen-presenting cell. Recognition of antigens involves direct binding of immunoglobulin to the intact antigen and antibodies typically bind to the surface of protein antigens. Cell mediated immunity is immune response that does not involve antibodies but rather incorporates the activation of macrophages and NK cells enabling them to destroy intracellular pathogens.
Humoral immunity is a form of immunity in which B lymphocytes and plasma cells produce antibodies to foreign agents (antigens) and stimulate T lymphocytes to attack them. Decline of immune response is a process associated with aging that leads to dysregulation of cells of innate and adaptive immunity, which may become dysfunctional. Immunologic memory is the ability of the immune system to respond more rapidly and effectively to pathogens that have been encountered previously, and reflects the pre-existence of a clonally expanded population of antigen-specific lymphocytes.
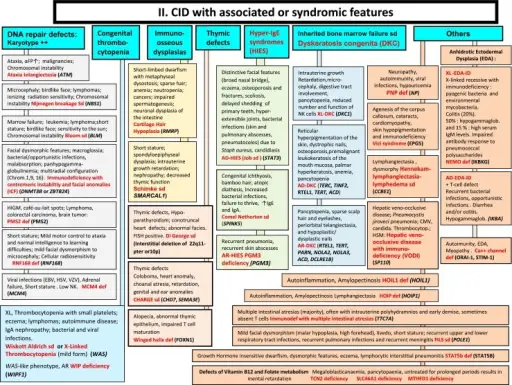
What are Primary Immunodeficiencies?
Primary immunodeficiencies involve an immune system that does not work correctly. People with primary immunodeficiencies are more likely to get and become severely ill from infections. Examples of primary immunodeficiencies include: severe combined immunodeficiency disease (SCID), ataxia-telangiectasia, selective IgA deficiency.
What are Defects in Innate Immunity?
Defects in innate immunity are that which blunt the response to infection. These defects may occur in monocytes, neutrophils, natural killer cells, basophils, mast cells or complement proteins. Examples of defects in innate immunity include toll-like receptor deficiencies, natural killer cell deficiency, defects in interferon gamma, and defects in interleukin 12.
What are Defects in the Complement System?
Defects in the complement system are immunodeficiencies of absent or suboptimal functioning of one of the complement system proteins. Examples of defects in the complement system include C1-inhibitor deficiency, C2 deficiency.
What are Defects in Adaptive Immunity?
Defects in adaptive immunity are failure of the recognition of specific “non-self” antigens, the failure of generation of pathogen-specific immunologic effector pathways and the failure of development of an immunologic memory. Examples of defects in adaptive immunity include IgG subclass deficiency, common variable immunodeficiency (CVID), transient hypogammaglobulinemia.
What are Defects in Lymphocyte Maturation?
Defects in lymphocyte maturation are failure of development of lymphocytes from their progenitor in bone marrow or thymus which result in decreased T cells or B cells. Examples of defects in lymphocyte maturation include autosomal severe combined immunodeficiency disease (SCID), recombination activating gene (RAG) deficiency.
What are Defects in Lymphocyte Activation and Function?
Defects in lymphocyte activation and function are failure to trigger antigen-specific receptors on the cell surface of lymphocytes. The failure to proliferation and differentiation into specialized cells results in defects. Examples of defects in lymphocyte activation include X-linked hyper-IgM syndrome, defective class II MHC expression, and T cell receptor defects.
What are Immunodeficiencies Associated with Systemic Diseases?
Immunodeficiencies associated with systemic diseases are those that are born with and that affect the entire body. Examples of immunodeficiencies associated with systemic diseases include Wiskott-Aldrich syndrome and ataxia telangiectasia.
What is X-linked Agammaglobulinemia aka Bruton Agammaglobulinemia?
Agammaglobulinemia aka Bruton agammaglobulinemia is an inherited (genetic) immune system disorder that reduces your ability to fight infections. Agammaglobulinemia is caused by a genetic mutation in a gene called Bruton’s tyrosine kinase.
What is the Pathology of X-linked Agammaglobulinemia?
The pathology of X-linked agammaglobulinemia is:
-Aetiology: The cause of disease X-linked agammaglobulinemia is a gene defect that blocks the growth of normal, mature immune cells called B lymphocytes.
-Pathogenesis: The sequence of events that lead to X-linked agammaglobulinemia are is Bruton tyrosine kinase deficiency causes a failure of B cell development in affected individuals. Immunoglobulin-secreting plasma cells also are absent, deficiency of immunoglobulins results in absent antibody responses and increases the tendency to develop bacterial infections.
-Morphologic changes in the X-linked agammaglobulinemia involves people with XLA have very small tonsils and lymph nodes. This is because tonsils and lymph nodes are made up of B lymphocytes. In people with XLA, the size of these tissues is reduced because of the absence of B lymphocytes.
How does X-linked Agammaglobulinemia Present?
Patients with X-linked agammaglobulinemia typically affects males because it is unlikely that females will have two altered copies of the gene because it is x-linked. Patients with XLA can clinically present when they are aged 3 months to 5 years because new born cannot produce their own Ig. The symptoms, features, and clinical findings associated with X-linked agammaglobulinemia include: nasal infections, skin infections, meningitis, bronchitis, pneumonia, sepsis, or infection of the blood stream.
How is X-linked Agammaglobulinemia Diagnosed?
X-linked agammaglobulinemia is diagnosed by electrophorphoresis. The diagnosis is confirmed by the absence of BTK protein in monocytes or platelets or by the detection of a mutation in BTK in DNA by using polymerase chain reaction (PCR)-based single-strand conformation polymorphism (SSCP).
How is X-linked Agammaglobulinemia Treated?
X-linked agammaglobulinemia has no cure, but the condition may be treated. Immunoglobulin replacement therapy is a life-long and life-prolonging treatment that restores some of the missing antibodies. Daily oral antibiotics are also utilized to prevent or treat infections.
What is the Prognosis of X-linked Agammaglobulinemia?
The prognosis of X-linked agammaglobulinemia is good as long as patients are diagnosed and treated early with regular intravenous gamma globulin therapy before the sequelae of recurrent infections appear.
What is Common Variable Immunodeficiency?
Common variable immunodeficiency (CVID) is an immune system disorder that causes to have low levels of the proteins that help fight infections. Patients with common variable immunodeficiency (CVID) are likely to have repeated infections in ears, sinuses and respiratory system.
What is the Pathology of Common Variable Immunodeficiency?
The pathology of common variable immunodeficiency is:
-Etiology: The cause of common variable immunodeficiency is mutations in the genes which result in dysfunctional B cells that cannot make sufficient amounts of antibodies. about 10 percent of cases, CVID is hereditary.
-Pathogenesis: The sequence of events that lead to common variable immunodeficiency defective B cell function leading to impaired immunoglobulin production causing recurrent infections, chronic lung disease, autoimmune disorders, gastrointestinal disease, or increased risk of malignancy.
-Morphologic changes: The morphologic changes involved with common variable immunodeficiency are immune dysregulations, malignancy, and recurrent infections.
How does Common Variable Immunodeficiency Present?
Patients with common variable immunodeficiency reported as earlier onset of symptoms and diagnosis in males however, common variable immunodeficiency (CVID) does not show any predilection for race or gender. Common variable immunodeficiency (CVID) is most frequently diagnosed in adults aged 20 to 40 years old. In some cases common variable immunodeficiency (CVID) may become obvious during childhood. The symptoms, features, and clinical findings associated with common variable immunodeficiency include: breathing issues, chronic cough, diarrhea, weight loss, frequent ear infections, and recurrent sinus infections.
How is Common variable immunodeficiency Diagnosed?
Common variable immunodeficiency (CVID) diagnosis can be made through screening tests that measure immunoglobulin levels or the number of B cells in the blood. immunofluorescence staining is mostly involved for laboratory tests of serum IgA, IgG and IgM levels which are decreased or absent in the disease.
How is Common Variable Immunodeficiency Treated?
Common variable immunodeficiency is treated by intravenous immunoglobulin infusions or subcutaneous (under the skin) immunoglobulin injection to partially restore immunoglobulin levels. The mainstay of treatment for common variable immunodeficiency (CVID) is Ig replacement therapy.
What is the Prognosis of Common Variable Immunodeficiency?
The prognosis of common variable immunodeficiency is reasonably good unless severe autoimmune disease or malignancy develops. There is no cure for common variable immunodeficiency (CVID). With ongoing treatment, many people with common variable immunodeficiency (CVID) live active and fulfilling lives. In some cases, complications of common variable immunodeficiency (CVID) such as lung damage or cancer may affect life expectancy. These complications appear over time. They may become life-threatening, but that process often takes years.
What is IgA Deficiency?
IgA deficiency is a disease is of low or absent immunoglobulin A (IgA). IgA is normally found in mucous membranes, mainly in the respiratory and digestive tracts. IgA is also normally found in saliva, tears, and breastmilk. A deficiency of IgA seems to play a part in asthma and allergies.
What is the Pathology of IgA Deficiency?
The pathology of IgA deficiency is:
-Etiology: The causes of IgA deficiency are intrinsic defects in B cells, anomalies in T-cell help, alterations of the cytokine network, and others. Drugs known to cause IgA deficiency include: Sulfasalazine, Phenytoin, Valproic acid, Thyroxine.
-Pathogenesis: The sequence of events that lead to IgA deficiency is a maturation defect in B cells to produce IgA. The defect appears to involve the stem cells since IgA deficiency can be transferred by bone marrow transplantation. The causes of selective IgA deficiency are unknown. IgA deficiency is likely due to a variety of causes, and this explains why the symptoms or health problems may vary widely from individual to individual.
-Morphologic changes: The morphologic changes involved with IgA deficiency are alterations in transmembrane activator and calcium modulator and cyclophilin ligand interactor gene. Major histocompatibility complex haplotypes have also been associated with susceptibility to IgA deficiency.
How does IgA Deficiency Present?
IgA deficiency disease typically involve males as compared to females. The symptoms features, and clinical findings associated with IgA deficiency include are very subtle, most people with an IgA deficiency don’t have any noticeable symptoms or the health problem. IgA deficiency is usually found on a blood test.
How is IgA Deficiency Diagnosed?
IgA deficiency is diagnosed by blood tests that check for an IgA deficiency with normal levels of other immunoglobulins.
How is IgA Deficiency Treated?
IgA deficiency has no specific treatment. Prophylactic antibiotics may be helpful in patients with recurrent sinopulmonary tract infections due to IgA deficiency.
What is the Prognosis of IgA Deficiency?
The prognosis of IgA deficiency is good in most cases. Respiratory infections and autoimmune disease associated with IgA deficiency may be symptomatically treated. A few patients with IgA deficiency presenting in childhood may recover spontaneously; other patients may develop common variable immunodeficiency (CVID).
What is Hyper-IgM Syndrome?
Hyper-IgM syndrome is an immunoglobulin deficiency syndrome that is characterized by normal or elevated serum IgM levels and decreased levels or absence of other serum immunoglobulins. Hyper-IgM syndrome can result in susceptibility to bacterial infections.
What is the Pathology of Hyper-IgM Syndrome?
The pathology of hyper-IgM syndrome is:
-Etiology: The cause of hyper-IgM syndrome is a variation in the CD40LG gene. Most cases (approximately 70%) of hyper-IgM syndrome are linked to a recessive mutation on the X chromosome. These cases are inherited as an X-linked recessive genetic trait.
-Pathogenesis: The sequence of events that lead to hyper-IgM syndrome are due to a variation in the CD40LG gene, which causes the body to not produce enough CD40 ligand, or produces an abnormal form of the CD40 ligand. Individuals with hyper-IgM syndrome lack functional levels of CD40 ligand, resulting in the B cell immune response that is deficient due to a result of a T cell defect.
-Morphologic changes: The morphologic changes involved with hyper-IgM syndrome are signs and symptoms of recurrent bacterial infections.
How does Hyper-IgM Syndrome Present?
Patients with hyper-IgM syndrome are typically in males because of the X-linked recessive inheritance pattern. Hyper-IgM syndrome may be diagnosed at very early age within one year. The symptoms, features, and clinical findings associated with hyper-IgM syndrome include frequent bacterial infections, especially bacterial pneumonia
How is Hyper-IgM Syndrome Diagnosed?
Hyper-IgM syndrome is diagnosed by blood tests to determine the status of immunoglobulins in the blood, including normal or high levels of IgM and low levels of other immunoglobulin classes. Flow cytometry may be used to aid in diagnosis, as well as genetic testing.
How is Hyper-IgM Syndrome Treated?
Hyper-IgM syndrome is treated by immune globulin replacement therapy. Patients with the X-linked form or CD40 mutations are given prophylactic antibiotics to prevent bacterial infections.
What is the Prognosis of Hyper-IgM Syndrome?
The prognosis of hyper-IgM syndrome is poor. Individuals with hyper-IgM syndrome are susceptible to various infections and cannot fight off infections well once they occur. Without proper treatment, these infections can become life-threatening.
What is Thymic Hypoplasia?
Thymic hypoplasia results in aberrant T-cell production, which negatively effects immune respnse. Thymic hypoplasia is also knowns as DiGeorge syndrome.
What is the Pathology of Thymic Hypoplasia?
The pathology of thymic hypoplasia aka DiGeorge syndrome is:
-Etiology: The cause of hypoplasia aka DiGeorge syndrome is a heterozygous deletion of part of the long arm (q) of chromosome 22, region 1, band 1, sub-band 2.
-Pathogenesis: The sequence of events that lead to thymic hypoplasia aka DiGeorge syndrome are abnormal development in the embryonic pharyngeal system. The T cell defects are the result of insufficient thymic tissue.
-Morphologic changes: The morphologic changes involved with thymic hypoplasia aka DiGeorge syndrome include: thymic hypoplasia, an abnormal chin, low set ears, and widely spaced apart eyes. Patients with thymic hypoplasia may also have cleft lips or cleft palates.
How does Thymic Hypoplasia Present?
Some signs and symptoms may be apparent at birth, but others may not appear until later in infancy or early childhood. The symptoms, features, and clinical findings associated with DiGeorge syndrome include: hearing and vision problems, mouth and feeding problems, delays in learning to walk or talk, short stature, frequent infections.
How is Thymic Hypoplasia Diagnosed?
Thymic hypoplasia or DiGeorge syndrome is diagnosed by fluorescent in situ hybridization (FISH) analysis which looks for the specific abnormal changes on chromosomes that cause the disease whcih is 22q11.2. Fluorescent in situ hybridization can be performed on cell samples obtained by amniocentesis as early as the fourteenth week of pregnancy. Diagnosis can be made by ultrasound examination around the eighteenth week of pregnancy, when abnormalities in the development of the heart or the palate can be detected.
How is Thymic Hypoplasia Treated?
Thymic hypoplasia aka DiGeorge syndrome is treated by a transplant of thymus tissue. This transplantation promotes T cell development. A cleft lip of cleft palate may be surgically repaired.
What is the Prognosis of Thymic Hypoplasia?
The prognosis of thymic hypoplasia aka DiGeorge syndrome depends on the severity of congenital disabilities. Some of these conditions may be life-threatening. With ongoing treatment and support, many individuals with DiGeorge syndrome may live active lives.
What is Severe Combined Immunodeficiency?
Severe combined immunodeficiency is an inherited primary immunodeficiency disease that typically presents in infancy results in profound immune deficiency condition resulting in a weak immune system that is unable to fight off even mild infections.
What is the Pathology of Severe Combined Immunodeficiency?
The pathology of severe combined immunodeficiency is:
-Etiology: The cause of severe combined immunodeficiency is genetic defects that affects the function of T cells. Depending on the type of SCID, B cells and NK cells can also be affected.
-Pathogenesis: The sequence of events that lead to severe combined immunodeficiency are due to decreased number of lymphocytes or defective lymphocytes. This results in a defective immune response, leaving the affected individual susceptible to many infections.
-Morphologic changes: The morphologic changes involved with severe combined immunodeficiency include restricted growth and failure to thrive.
How does Severe Combined Immunodeficiency Present?
Patients with severe combined immunodeficiency are typically male due to the X-linked pattern of inheritance. Patients with severe combined immunodeficiency typically present by six months of age with infections by opportunistic pathogens. The symptoms, features, and clinical findings associated with severe combined immunodeficiency include frequent infections.
How is Severe Combined Immunodeficiency Diagnosed?
Severe combined immunodeficiency is diagnosed by a complete medical history and physical examination of the individual. Blood and genetic tests may help confirm the diagnosis.
How is Severe Combined Immunodeficiency Treated?
Severe combined immunodeficiency is treated by stem cell transplantation with immune cells that may have the ability to grow in the recipients body to ward off potential infections.
What is the Prognosis of Severe Combined Immunodeficiency?
The prognosis of severe combined immunodeficiency is poor. Infants with SCID usually die from infections within the first two years of life. After a successful stem cell transplant they may have an better of living a healthier life.
What is Wiskott-Aldrich Syndrome?
Wiskott–Aldrich syndrome is a rare X-linked recessive disease characterized by eczema, thrombocytopenia (low platelet count), immune deficiency, and bloody diarrhea.
What is the Pathology of Wiskott–Aldrich Syndrome?
The pathology of Wiskott-Aldrich syndrome is:
-Etiology: The cause of Wiskott-Aldrich syndrome is a defect in the WAS gene located on the short arm of the X-chromosome at the Xp 11.22-23 position.
-Pathogenesis: The sequence of events that lead to Wiskott-Aldrich syndrome are a genetic mutation in the gene encoding Wiskott-Aldrich syndrome protein (WASp) affecting the immune system and inducing a state of immunodeficiency.
-Morphologic changes: The morphologic changes involved with wiskott–aldrich syndrome include eczema, reduced platelet size and function, and immunodeficiency with decreased T lymphocyte function, and an inability to make antibodies to carbohydrate antigens.
How does Wiskott–Aldrich Syndrome Present?
Patients with Wiskott-Aldrich syndrome are typically males. Age at presentation ranges from birth to 25 years old. The symptoms, features, and clinical findings associated with Wiskott-Aldrich syndrome include: eczema, frequent nose bleeds, chronic infections, anemia, arthritis, inflammatory bowel disease, nephritis, and vasculitis.
How is Wiskott–Aldrich Syndrome Diagnosed?
Wiskott–Aldrich syndrome is diagnosed by a test that measures the amount of platelets. a genetic test that reveals presence of a mutation in the Wiskott-Aldrich syndrome gene. a blood test that demonstrates absence of the Wiskott-Aldrich syndrome protein in the white blood cells. Tests include gene sequencing, flow cytometry, quantitative immunoglobulins levels, complete blood counts.
How is Wiskott–Aldrich Syndrome Treated?
Wiskott–Aldrich syndrome is treated by stem cell transplant (also known as a bone marrow transplant). Antibody infusions because B cells may not produce antibodies against infections.
What is the Prognosis of Wiskott–Aldrich Syndrome?
The prognosis of Wiskott–Aldrich syndrome is poor. The average age of survival is 15 years old.
What is Ataxia Telangiectasia?
Ataxia telangiectasia is a rare inherited disorder that affects the nervous system, immune system, and other body systems. This disorder is characterized by progressive difficulty with coordinating movements.
What is the Pathology of Ataxia Telangiectasia?
The pathology of ataxia telangiectasia is:
-Etiology: The cause of ataxia telangiectasia is caused by changes (mutations) of a gene known as A
-Pathogenesis: The sequence of events that lead to ataxia telangiectasia are involve mutations of the ATM gene product, ATM kinase. ATM kinase is involved in the detection of DNA damage and plays an important role in cell cycle progression. Without a properly forming ATM kinase, immune and nervous system dysfunctions develop.
-Morphologic changes: The morphologic changes involved with ataxia telangiectasia are ataxia and telangiectasis. Ataxia is progressively impaired coordination of voluntary movements. Telangiectasis are vascular lesions that may be visible on the skin and mucous membranes due to permanent widening of groups of blood vessels.
How does Ataxia Telangiectasia Present?
Patients with ataxia telangiectasia has no gender predilection. Males and females may be affected in equal numbers. Ataxia telangiectasia may begin present during infancy. The symptoms, features, and clinical findings associated with ataxia telangiectasia include: recurrent infections, decreased mental development, delayed walking, abnormal eye movements, red discoloration of the skin in sun exposed areas, and seizures.
How is Ataxia Telangiectasia Diagnosed?
Ataxia telangiectasia is diagnosed by genetic tests to assess for mutations in the ATM gene.
How is Ataxia Telangiectasia Treated?
Ataxia telangiectasia has no specific treatment, and symptoms are managed individually.
What is the Prognosis of Ataxia Telangiectasia?
The prognosis of ataxia telangiectasia is good, however it is a multi-organ neurodegenerative disorder with enhanced vulnerability to cancer and infection.
What are Secondary Immunodeficiencies?
Secondary immunodeficiencies are the result of disease or other environmental factors weakening the immune system. A prime examples of a secondary immunodeficiency disorder is acquired immunodeficiency syndrome (AIDS).
What is AIDS?
AIDS is acquired immunodeficiency syndrome. AIDS is a chronic, potentially life-threatening condition caused by the human immunodeficiency virus (HIV). HIV interferes with the ability to fight infections.
What is the Pathology of AIDS?
The pathology of AIDS is:
-Etiology: The cause of AIDS due to a breakdown of the immune system, a result of the fact that HIV infects and destroys a specialized group of white blood cells called T-helper of T-4 cells.
-Pathogenesis: The sequence of events that lead to AIDS are due to defective innate signaling pathways, increased viral replication and increased viral load. AIDS results in gradual loss of peripheral CD4+ T cells and depletion of T lymphocytes at mucosal sites that collectively lead to progressive immune deficiency and AIDS.
-Morphologic changes: The morphologic changes involved with AIDS include increased deposition of adipose tissue, or weight loss.
How does AIDS Present?
Patients with AIDS typically women are more susceptible to HIV infection than men, present at age range of 15 to 49 years old. The symptoms, features, and clinical findings associated with AIDS include: infections, fatigue, genital sores, anal sores, white spots in the mouth, fevers, and weight loss.
How is AIDS Diagnosed?
AIDS is diagnosed by blood tests which look for antibodies to the HIV-virus that are present in the blood of infected individuals. An enzyme linked immuno-assay test or polymerase chain reaction test are used to diagnose AIDS.
How is AIDS Treated?
AIDS is treated by antiretroviral therapy, a combination of daily medications that stop the virus from reproducing. This helps protect CD4 cells, keeping the immune system strong enough to take measures against disease.
What is the Prognosis of AIDS?
The prognosis of AIDS is in patients with untreated HIV infection is poor without proper treatment. Without treatment the average time from infection to death is 8-10 years.
Table of Immunodeficiency Disorders
| No. | Disease | Causes | Symptoms | Diagnosis | Treatment | Prognosis |
| 1 | X-linked Agammaglobulinemia | Bruton tyrosine kinase deficiency causes a failure of B cell development | meningitis, bronchitis, pneumonia, sepsis, infections | PCR)-based single-strand conformation polymorphism (SSCP). | Immunoglobulin replacement therapy, oral antibiotics to treat infections | good |
| 2 | Common Variable Immunodeficiency | Mutations in the genes which result in dysfunctional B cells | Breathing problems, chronic cough, diarrhoea, lung infections, pneumonia | immunofluorescence staining, | Ig replacement therapy | reasonably good |
| 3 | IgA Deficiency | intrinsic defects in B cells, anomalies in Th-cell, | frequent infections or NO symptoms | blood screening for IgA levels | Prophylactic antibiotics for infections | good |
| 4 | Hyper-IgM Syndrome | a variation in the CD40LG gene, X-linked recessive mutation | Tachypnea, bluish discoloration (cyanosis) of the skin | flow cytometry, molecular genetic testing | immunoglobulin replacement therapy | Poor |
| 5 | DiGeorge Syndrome | deletion of part of the long arm of chromosome 22 | underdeveloped chin, low-set ears, wide-set eyes or a narrow groove in the upper lip | FISH analysis | thymus tissue transplant, abnormalities surgically repaired, antibiotics | Depends on severity |
| 6 | Severe Combined Immunodeficiency | genetic defects affecting the function of T cells | frequent, often serious respiratory infections, chronic diarrhoea | multiple blood tests, flow cytometry, DNA sequencing | bone marrow transplant | Poor |
| 7 | Wiskott–Aldrich syndrome | defect in the WAS gene | eczema arthritis, inflammatory bowel disease, nephritis, vasculitis | Blood tests, DNA sequencing, flow cytometry, quantitative Ig levels | stem cell transplant, antibiotics | Poor |
| 8 | Ataxia Telangiectasia | ATM gene Mutation on chromosome 11 | Decreasing mental development, Seizures, Severe respiratory infections recurring | Serum Ig levels, gene sequencing, X-rays | symptomatic treatments | good |
| 9 | AIDS | human immunodeficiency virus (HIV) | severe fatigue, genital or anal sore, regular coughing and breathing problems, fever | ELISA Test | antiretroviral therapy to protect CD4 cells | Poor |