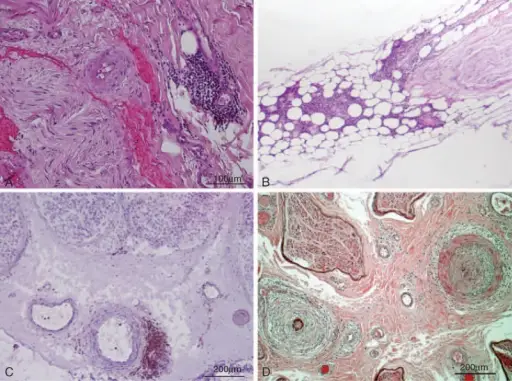
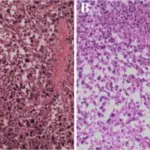
Peripheral nervous system pathology is any disease or disorder that affects the peripheral nervous system.
Examples of peripheral nerve pathology include:
- Diseases of peripheral nerves
- Inflammatory neuropathies
- Infectious neuropathies
- Hormonal neuropathies
- Metabolic and nutritional neuropathies
- Toxic neuropathies
- Malignancy associated neuropathies
- Neuropathies caused by physical forces
- Inherited peripheral neuropathies
- Diseases of the neuromuscular junction
- Peripheral nerve sheath tumors
What are Diseases of Peripheral Nerves?
Diseases of peripheral nerves are the conditions affecting functional elements of peripheral nerves which include axonal processes and their myelin sheaths. These diseases may cause changes to the normal anatomical structure of peripheral nerves.
Examples of diseases of peripheral nerves include:
- Axonal neuropathies
- Demyelinating neuropathies
- Neuronopathies
What are Axonal Neuropathies?
Axonal neuropathies are the destruction of the axonal structure of the nerve. Axons are damaged by direct insult.
What are Demyelinating Neuropathies?
Demyelinating neuropathies are the destruction of the Schwann cells. Myelin is damaged with sparing of the axon resulting in abnormally slow nerve conduction velocities.
What are Neuronopathies?
Neuronopathies are generalized abnormalities affecting the neuron cells. Destruction of neurons leads to secondary degeneration of axonal processes.
What are the Patterns of Peripheral Neuropathies?
Patterns of peripheral neuropathies are the anatomical pattern causing either sensory or motor damage or both.
Examples of patterns of peripheral neuropathies include:
- Mononeuropathies: Single nerve affected.
- Polyneuropathies: Multiple nerves affected.
- Mononeuritis multiplex: Several nerves damaged in a haphazard manner.
- Polyradiculoneuropathies: Diffuse symmetric symptoms due to nerve roots and peripheral nerves being affected.
What are Inflammatory Neuropathies?
Inflammatory neuropathies are the diseases of the neurons characterized by peripheral nerves, roots, and sensory and autonomic ganglia inflammatory cell infiltrates.
Examples of inflammatory neuropathies include:
- Acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome)
- Chronic inflammatory demyelinating polyneuropathy
- Neuropathy associated with systemic autoimmune diseases
- Neuropathy associated with vasculitis
What is Acute Inflammatory Demyelinating Polyneuropathy (Guillain-Barre Syndrome)?
Acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome) is a life-threatening, swiftly progressive acute demyelinating ailment which affects the motor axons.
What is the Pathology of Acute Inflammatory Demyelinating Polyneuropathy (Guillain-Barre Syndrome)?
The pathology of acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome) is quick progressive acute demyelinating ailment affecting motor axons.
-Etiology: The cause of acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome) is the post-infection involving the peripheral nerves. Infectious agents include: Influenza, Epstein-Barr virus, Campylobacter jejuni, cytomegalovirus, and Mycoplasma pneumoniae.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to acute inflammatory demyelinating polyneuropathy (guillain-barre syndrome) results from cell-mediated immune response, convoyed by segmented demyelination brought by activated macrophages produced during the infection.
-Morphology: The morphology associated with acute inflammatory demyelinating polyneuropathy (guillain-barre syndrome) shows inflammation of the peripheral nerve.
-Histology: The histology associated with acute inflammatory demyelinating polyneuropathy (guillain-barre syndrome) shows endoneurial/perivenular lymphocytes and macrophages infiltration.
How does Acute Inflammatory Demyelinating Polyneuropathy (Guillain-Barre Syndrome) Present?
Patients with acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome) typically have male preponderance of older patients present at an age range of infancy and old age. The symptoms, features, and clinical findings associated with acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome) include weakness, paralysis, and absence deep tendon reflexes.
How is Acute Inflammatory Demyelinating Polyneuropathy (Guillain-Barre Syndrome) Diagnosed?
Acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome) through clinical presentations. Laboratory studies such as metabolic panels and CBCs, to exude other causes. Nerve conduction studies, and electromyography. peripheral neuropathy workup. MRIs disclose nerve root enhancement.
How is Acute Inflammatory Demyelinating Polyneuropathy (Guillain-Barre Syndrome) Treated?
Acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome) is treated through hospitalization (ICU) for close monitoring. Medical care- Immunomodulatory treatment and corticosteroids therapy.
What is the Prognosis of Acute Inflammatory Demyelinating Polyneuropathy (Guillain-Barre Syndrome)?
The prognosis of acute inflammatory demyelinating polyneuropathy (Guillain-Barre syndrome) is fair with a 2-12% mortality rate.
What is Chronic Inflammatory Demyelinating Polyneuropathy?
Chronic inflammatory demyelinating polyneuropathy is an immune-mediated inflammation of the neurons manifesting as a symmetric demyelinating disease.
What is the Pathology of Chronic Inflammatory Demyelinating Polyneuropathy?
The pathology of chronic inflammatory demyelinating polyneuropathy is:
-Etiology: The cause of chronic inflammatory demyelinating polyneuropathy is immune-mediated.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to chronic inflammatory demyelinating polyneuropathy results from cell-mediated immune response, convoyed by segmented demyelination brought by activated macrophages produced during the infection.
-Morphology: NA.
-Histology: The histology associated with chronic inflammatory demyelinating polyneuropathy shows endoneurial/perivenular lymphocytes and macrophages infiltration.
How does Chronic Inflammatory Demyelinating Polyneuropathy Present?
Patients with chronic inflammatory demyelinating polyneuropathy typically affect both sexes present at an age range of any age. The symptoms, features, and clinical findings associated with chronic inflammatory demyelinating polyneuropathy include limb weakness, hands and feet tingling and numbness, orthostatic dizziness, abnormal gait, and deep tendon reflexes absent.
How is Chronic Inflammatory Demyelinating Polyneuropathy Diagnosed?
Chronic inflammatory demyelinating polyneuropathy is diagnosed through clinical presentations, laboratory studies which include CBCs, ESR, biochemical profile, urine immune-electrophoresis, and antinuclear antibody (ANA) level. Imaging studies- spine MRI, electromyography (EMG) and peripheral nerve biopsy.
How is Chronic Inflammatory Demyelinating Polyneuropathy Treated?
Chronic inflammatory demyelinating polyneuropathy is treated through plasmapheresis and administration of immunosuppressive agents.
What is the Prognosis of Chronic Inflammatory Demyelinating Polyneuropathy?
The prognosis of chronic inflammatory demyelinating polyneuropathy is fair, complete recovery but more frequently recurring bouts of symptomatic disease.
What is Neuropathy Associated with Systemic Autoimmune Diseases?
Neuropathy associated with systemic autoimmune diseases is a group of immune-mediated neuropathies that sporadically present in patients with systemic autoimmune ailments.
What is the Pathology of Neuropathy Associated with Systemic Autoimmune Diseases?
The pathology of neuropathy associated with systemic autoimmune diseases is:
-Etiology: The cause of neuropathy associated with systemic autoimmune diseases is ill regulation of innate and adaptive immunity.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to neuropathy associated with systemic autoimmune diseases results from an impairment of the nutrient supply of peripheral nerves by neuronal cell body and blood vessels causing malfunctioning.
-Morphology: NA.
-Histology: NA.
How does Neuropathy Associated with Systemic Autoimmune Diseases Present?
Patients with neuropathy associated with systemic autoimmune diseases typically have no gender prevalence present at the age range of any age. The symptoms, features, and clinical findings associated with neuropathy associated with systemic autoimmune diseases include paresthesia, malaise, weight loss, fever, night sweats,
How is Neuropathy Associated with Systemic Autoimmune Diseases Diagnosed?
Neuropathy associated with systemic autoimmune diseases is diagnosed through clinical presentations. Cerebrospinal fluid (CSF) analysis, CBCs, biochemical profile, urine immune-electrophoresis may be helpful. Other studies such as spine MRI, electromyography (EMG) and peripheral nerve biopsy may be warranted.
How is Neuropathy Associated with Systemic Autoimmune Diseases Treated?
Neuropathy associated with systemic autoimmune diseases is treated by managing the underlying condition.
What is the Prognosis of Neuropathy Associated with Systemic Autoimmune Diseases?
The prognosis of neuropathy associated with systemic autoimmune diseases is good, with proper management of the underlying condition.
What is Neuropathy Associated with Vasculitis?
Neuropathy associated with vasculitis is part of systemic vasculitis presenting with either mononeuritis multiplex or asymmetric sensorimotor neuropathy.
What is the Pathology of Neuropathy Associated with Vasculitis?
The pathology of neuropathy associated with vasculitis is:
-Etiology: The cause of neuropathy associated with vasculitis is blood vessel defects.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to neuropathy associated with vasculitis results after ischemic infarction instigated by inflammatory constriction of the blood vessels.
-Morphology: NA.
-Histology: NA.
How does Neuropathy Associated with Vasculitis Present?
Patients with neuropathy associated with vasculitis typically affect both genders equally present at age range 30 to 60 years. The symptoms, features, and clinical findings associated with neuropathy associated with vasculitis include myalgias, fevers, weight loss, fatigue, arthralgias, anorexia, and poorly localized acute pain.
How is Neuropathy Associated with Vasculitis Diagnosed?
Neuropathy associated with vasculitis is diagnosed through laboratory studies-ESR> 20 mm/h, elevated antinuclear antibody titer, rheumatoid factor, renal function test, serum complement. Other studies that may be helpful include imaging studies, electromyography, and nerve conduction studies.
How is Neuropathy Associated with Vasculitis Treated?
Neuropathy associated with vasculitis is treated through medical care- immunosuppressive agent, corticosteroids and cyclophosphamide therapy.
What is the Prognosis of Neuropathy Associated with Vasculitis?
The prognosis of neuropathy associated with vasculitis is poor.
What are Infectious Neuropathies?
Infectious neuropathies are neuron injuries and damage due to the presence of an infectious disease.
Examples of infectious neuropathies include:
- Leprosy (Hansen disease) infectious neuropathy
- Diphtheria infectious neuropathy
- HIV/AIDS infectious neuropathy
- Lyme disease infectious neuropathy
- Varicella-Zoster virus infectious neuropathy
What is Leprosy (Hansen Disease) Infectious Neuropathy?
Leprosy (Hansen disease) infectious neuropathy is a chronic curable cause of neuropathy infection caused by the Mycobacterium leprae.
What is the Pathology of Leprosy (Hansen Disease) Infectious Neuropathy?
The pathology of leprosy (Hansen disease) infectious neuropathy is:
-Etiology: The cause of leprosy (Hansen disease) infectious neuropathy is Mycobacterium leprae.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to leprosy (Hansen disease) infectious neuropathy results from Schwann cells attacked by Mycobacterium leprae. This results in inflammation that injures the cutaneous nerves.
-Morphology: NA.
-Histology: The histology associated with leprosy (Hansen disease) infectious neuropathy shows nerve bundles with inflation, mononuclear cells, and granulomas.
How does Leprosy (Hansen Disease) Infectious Neuropathy Present?
Patients with leprosy (Hansen disease) infectious neuropathy are typically more common in males than females present at an age range of any age. The symptoms, features, and clinical findings associated with leprosy (Hansen disease) infectious neuropathy include painless, non-itchy skin patches, diminished/loss of sensation, trophic ulcers and blisters, progressive wasting and weakness.
How is Leprosy (Hansen Disease) Infectious Neuropathy Diagnosed?
Leprosy (Hansen disease) infectious neuropathy is diagnosed through clinical presentations: laboratory studies- Skin biopsy to assess for acid-fast bacilli. Serologic assays detect phenolic glycolipid-1 and lipoarabinomannan.
How is Leprosy (Hansen Disease) Infectious Neuropathy Treated?
Leprosy (Hansen disease) infectious neuropathy is treated through medical management which may include multidrug regimen therapy (rifampicin, dapsone, and clofazimine). Surgery may be indicated.
What is the Prognosis of Leprosy (Hansen Disease) Infectious Neuropathy?
The prognosis of leprosy (Hansen disease) infectious neuropathy is fair with proper management.
What is Diphtheria Infectious Neuropathy?
Diphtheria infectious neuropathy is an acute demyelinating polyneuropathy, peripheral nerve involvement resulting from the effects of the diphtheria exotoxin.
What is the Pathology of Diphtheria Infectious Neuropathy?
The pathology of diphtheria infectious neuropathy is:
-Etiology: The cause of diphtheria infectious neuropathy is diphtheria exotoxin
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to diphtheria infectious neuropathy results from diphtheria exotoxin effects on the peripheral nerves- selective demyelination of axons.
-Morphology: NA.
-Histology: NA.
How does Diphtheria Infectious Neuropathy Present?
Patients with diphtheria infectious neuropathy typically have no gender predilection present at age range of any age. The symptoms, features, and clinical findings associated with diphtheria infectious neuropathy include orthostatic hypotension, facial pallor, anhidrosis, hypohidrosis pruritus, dysesthesia, allodynia, and hyperalgesia. Peripheral nerve affected by diphtheria exotoxin characterized by loss of vibratory sensation and proprioception.
How is Diphtheria Infectious Neuropathy Diagnosed?
Diphtheria infectious neuropathy is diagnosed through the clinical presentation, laboratory studies such as immunoelectrophoresis, basic metabolic panel, LFTs. Imaging studies may also be useful.
How is Diphtheria Infectious Neuropathy Treated?
Diphtheria infectious neuropathy is treated through pharmacologic therapy, immunomodulatory therapy.
What is the Prognosis of Diphtheria Infectious Neuropathy?
The prognosis of diphtheria infectious neuropathy is fair, with proper management.
What is HIV/AIDS Infectious Neuropathy?
HIV/AIDS Infectious neuropathy is the most recurrent neurological complication of HIV infection.
What is the Pathology of HIV/AIDS Infectious Neuropathy?
The pathology of HIV/AIDS infectious neuropathy is:
-Etiology: The cause of HIV/AIDS infectious neuropathy is HIV complication.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to HIV/AIDS infectious neuropathy results from the effect of HIV on the various motor and sensory nerves of distal parts of the limbs, causing HIV polyneuropathy.
-Morphology: NA.
-Histology: NA.
How does HIV/AIDS Infectious Neuropathy Present?
Patients with HIV/AIDS infectious neuropathy typically have no gender preference present at the age range of any age. The symptoms, features, and clinical findings associated with HIV/AIDS infectious neuropathy include paresthesias, pain at the hands and feet, numbness, and feet and hands muscles weakness.
How is HIV/AIDS Infectious Neuropathy Diagnosed?
HIV/AIDS infectious neuropathy is diagnosed through clinical presentations, electromyography, nerve conduction studies, skin biopsies to evaluate cutaneous nerve innervation.
How is HIV/AIDS Infectious Neuropathy Treated?
HIV/AIDS infectious neuropathy is treated by good control of the HIV infection, anti-seizure medications, analgesics, antidepressants therapy for neurologic pain. Treat accordingly for GBS due to HIV.
What is the Prognosis of HIV/AIDS Infectious Neuropathy?
The prognosis of HIV/AIDS infectious neuropathy is fair. The condition recurs frequently.
What is Lyme Disease Infectious Neuropathy?
Lyme disease infectious neuropathy is a rare neurological syndrome caused by untreated lyme disease.
What is the Pathology of Lyme Disease Infectious Neuropathy?
The pathology of lyme disease infectious neuropathy is:
-Etiology: The cause of lyme disease infectious neuropathy is the untreated lyme disease.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to lyme disease infectious neuropathy results from the complication of the untreated lyme disease affecting the peripheral nerves.
-Morphology: NA.
-Histology: NA.
How does Lyme Disease Infectious Neuropathy Present?
Patients with lyme disease infectious neuropathy typically have no gender favorite present at any age range of any age. The symptoms, features, and clinical findings associated with lyme disease infectious neuropathy include tingling sensation, numbness, muscle weakness and loss of control to the foot.
How is Lyme Disease Infectious Neuropathy Diagnosed?
Lyme disease infectious neuropathy is diagnosed through clinical presentations, enzyme-linked immunoassay (ELISA), spinal tap electromyography (EMG), and brain MRI.
How is Lyme Disease Infectious Neuropathy Treated?
Lyme disease infectious neuropathy is treated through PO/IV antibiotics therapy. Physical therapy is also recommended.
What is the Prognosis of Lyme Disease Infectious Neuropathy?
The prognosis of lyme disease infectious neuropathy is good with proper management.
What is Varicella-Zoster Virus Infectious Neuropathy?
Varicella-Zoster virus infectious neuropathy is a common viral infection to the peripheral nervous symptoms.
What is the Pathology of Varicella-Zoster Virus Infectious Neuropathy?
The pathology of varicella-zoster virus infectious neuropathy is:
-Etiology: The cause of varicella-zoster virus infectious neuropathy is Varicella-Zoster Virus Infectious complication.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to varicella-zoster virus infectious neuropathy results from viral infections of the peripheral nervous system. Latent infection of neurons in the sensory ganglia of the spinal cord and brain stem follows chickenpox. Reactivation causes painful, vesicular skin outbreaks at the site of sensory dermatomes.
-Morphology: The morphology associated with varicella-zoster virus infectious neuropathy shows necrosis and hemorrhage at the site.
-Histology: The histology associated with varicella-zoster virus infectious neuropathy shows inflammatory insinuates of mononuclear.
How does Varicella-Zoster Virus Infectious Neuropathy Present?
Patients with varicella-zoster virus infectious neuropathy typically have gender prevalence present at an age range of childhood. The symptoms, features, and clinical findings associated with varicella-zoster virus infectious neuropathy include peripheral facial weakness, external auditory canal rush.
How is Varicella-Zoster Virus Infectious Neuropathy Diagnosed?
Varicella-zoster virus infectious neuropathy is diagnosed through the clinical presentation, laboratory studies, CSF analysis presence of anti-VZV antibodies.
How is Varicella-Zoster Virus Infectious Neuropathy Treated?
Varicella-zoster virus infectious neuropathy is treated through intravenous acyclovir therapy, VZV immune globulin, and VZV vaccine.
What is the Prognosis of Varicella-Zoster Virus Infectious Neuropathy?
The prognosis of varicella-zoster virus infectious neuropathy is fair.
What are Hormonal Neuropathies?
Hormonal neuropathies are neuron damage due to disrupted normal metabolic caused by endocrine disorders resulting in hormonal imbalances.
An example of hormonal neuropathy is thyroid dysfunction hormonal neuropathy.
What is Thyroid Dysfunction Hormonal Neuropathy?
Thyroid dysfunction hormonal neuropathy is uncommon neuropathy associated with severe, long-term, untreated hypothyroidism.
What is the Pathology of Thyroid Dysfunction Hormonal Neuropathy?
The pathology of thyroid dysfunction hormonal neuropathy is:
-Etiology: The cause of thyroid dysfunction hormonal neuropathy is unknown.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to thyroid dysfunction hormonal neuropathy is unknown.
-Morphology: NA.
-Histology: NA.
How does Thyroid Dysfunction Hormonal Neuropathy Present?
Patients with thyroid dysfunction hormonal neuropathy are typically more common in females than males present at an age range of 30 to 60 years. The symptoms, features, and clinical findings associated with thyroid dysfunction hormonal neuropathy include burning sensation, pain, numbness and tingling sensation.
How is Thyroid Dysfunction Hormonal Neuropathy Diagnosed?
Thyroid dysfunction hormonal neuropathy is diagnosed through clinical presentation and laboratory studies-TFTs.
How is Thyroid Dysfunction Hormonal Neuropathy Treated?
Thyroid dysfunction hormonal neuropathy is treated through medical care of the underlying hypothyroidism and symptoms. Levothyroxine.
What is the Prognosis of Thyroid Dysfunction Hormonal Neuropathy?
The prognosis of thyroid dysfunction hormonal neuropathy is good with proper hypothyroidism management and symptoms.
What are Metabolic and Nutritional Neuropathies?
Metabolic and nutritional neuropathies are neuron damages associated with metabolic disorders and nutritional disorders.
Examples of metabolic and nutritional neuropathies include:
- Diabetic neuropathy
- Uremic neuropathy
- Neuropathy due to vitamin B12 deficiency
What is Diabetic Neuropathy?
Diabetic neuropathy is a common complication of diabetes mellitus.
What is the Pathology of Diabetic Neuropathy?
The pathology of diabetic neuropathy is:
-Etiology: The cause of diabetic neuropathy is long-standing diabetes.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to diabetic neuropathy is not completely understood.
-Morphology: NA.
-Histology: The morphology associated with diabetic neuropathy shows thickening, hyalinization and endoneurial arterioles.
How does Diabetic Neuropathy Present?
Patients with diabetic neuropathy typically have equal frequency present at an age range of any age. The symptoms, features, and clinical findings associated with diabetic neuropathy include numbness, loss of balance, prickling pain, tingling sensation, aching, tightness, muscle weakness of the extremities, and gait instability.
How is Diabetic Neuropathy Diagnosed?
Diabetic neuropathy is diagnosed through the clinical presentation, laboratory studies- hemoglobin A1c and fasting plasma glucose. Imaging studies- MRI of cervical, lumbar, thoracic, regions exclude another cause. Needle electromyography.
How is Diabetic Neuropathy Treated?
Diabetic neuropathy is treated through the management of diabetes. Medical care including glycemic control, antidepressants and anticonvulsant drugs for neuropathic pain may be needed.
What is the Prognosis of Diabetic Neuropathy?
The prognosis of diabetic neuropathy is good, but the quality of life is reduced.
What is Uremic Neuropathy?
Uremic neuropathy is the distal sensorimotor polyneuropathy instigated by uremic toxins presence.
What is the Pathology of Uremic Neuropathy?
The pathology of uremic neuropathy is:
-Etiology: The cause of uremic neuropathy is renal inadequacy
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to uremic neuropathy is unclear.
-Morphology: The morphology associated with uremic neuropathy shows axonal degeneration
-Histology: NA.
How does Uremic Neuropathy Present?
Patients with uremic neuropathy typically more common in males than females present at an age range of any age. The symptoms, features, and clinical findings associated with uremic neuropathy include lower extremities tingling, prickling sensation and weakness, muscle cramps, paraesthesia, pain sensation, diminished vibratory perception, deep tendon reflexes absent.
How is Uremic Neuropathy Diagnosed?
Uremic neuropathy is diagnosed clinical presentations, laboratory studies- Nerve conduction study, cerebrospinal fluid analysis protein elevated, creatinine clearance <10mL/min,
How is Uremic Neuropathy Treated?
Uremic neuropathy is treated through hemo/peritoneal dialysis management of uremia. Surgical care- renal transplant.
What is the Prognosis of Uremic Neuropathy?
The prognosis of uremic neuropathy is good with hemo- or peritoneal dialysis management of uremia.
What is Neuropathy due to Vitamin B12 Deficiency?
Neuropathy due to vitamin B12 deficiency is the damage of the myelin sheath caused by lack of cobalamin.
What is the Pathology of Neuropathy due to Vitamin B12 Deficiency?
The pathology of neuropathy due to vitamin B12 deficiency is:
-Etiology: The cause of Neuropathy due to Vitamin B12 deficiency is Nutrition factors, metabolic factors.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to neuropathy due to vitamin B12 deficiency, the cause of damage of the myelin sheath triggered by cobalamin deficiency remains unclear.
-Morphology: NA.
-Histology:
How does Neuropathy due to Vitamin B12 Deficiency Present?
Patients with neuropathy due to vitamin B12 deficiency are typically higher in females than males present at an age range of 40 to 70 years. The symptoms, features, and clinical findings associated with Neuropathy due to Vitamin B12 deficiency include poor coordination, sensory loss, numbness, pain, tingling sensation of the feet and hand, and weakness.
How is Neuropathy due to Vitamin B12 Deficiency Diagnosed?
Neuropathy due to vitamin B12 deficiency is diagnosed through clinical presentations, laboratory studies such as nerve conduction velocity test, blood test. Imaging- electromyography.
How is Neuropathy due to Vitamin B12 Deficiency Treated?
Neuropathy due to vitamin B12 deficiency is treated through medical care that may include B12 therapy, and nutritional modification.
What is the Prognosis of Neuropathy due to Vitamin B12 Deficiency?
The prognosis of neuropathy due to Vitamin B12 deficiency is good. Early diagnosis and treatment reverse the nerve dysfunction.
What are Toxic Neuropathies?
Toxic neuropathies are alteration of the nervous system function that is caused by toxins from either medication or drugs or other substances.
Examples of toxic neuropathies include:
- Alcohol neuropathy
- Arsenic neuropathy
- Lead neuropathy
- Mercury neuropathy
- Taxane neuropathy
- Thallium neuropathy
- Vinca alkaloids neuropathy
| EXAMPLES OF TOXIC NEUROPATHIES. | |||
| NEUROPATHY | SIGNS/SYMPTOMS | TREATMENT | PROGNOSIS |
| Alcohol neuropathy | immobility, loss of sensation, muscle weakness, tingling sensation, constipation, diarrhea | Alcohol cessation, detox therapy, rehab assistance, vitamins, pain medication, and physical therapy. | Good if one adheres to the process of rehabilitation and cessation of alcohol. |
| Arsenic neuropathy | Muscle pain, fatigue, confusion, memory loss | Chelating agents, dialysis, bowel cleansing, blood transfusion | Good since it has a low mortality rate |
| Lead neuropathy | Anorexia constipation, numbness, dizziness, azotemia, osteomalacia, anemia, muscle weakness. | Chelation therapy, | Good with a very low mortality rate because it responds well to chelation therapy. |
| Mercury neuropathy | Metal fume fever consists of painful ejaculation, fever, chills, dizziness, headache, acrodynia, erethism. | Chelation therapy, removal of the source of mercury, supportive therapy, dietary therapy. | Fair but the damage that is usually caused by mercury poisoning is usually severe. |
| Taxane neuropathy | Tingling sensation, pain, numbness, | Gradual reduction of the dosage. | |
| Thallium neuropathy | Alopecia, stomach ache, nausea, vomiting, numbness, tingling sensation, | Antidote with Radiogardase, removal of the source, maintain oxygen patency, | Is fair because the chances of death are low but it can lead to dementia and psychosis. |
| Vinca alkaloids neuropathy | Loss of tendon function, numbness, constipation. | antidote hyaluronidase, | Good since the only long-term effects that occur can vanish after continuous treatment. |
Table showing toxic neuropathies.
What are Malignancy Associated Neuropathies?
Malignancy associated neuropathies are diseases of the nervous system that occur as a result of pre-existing malignancies in the individual and the medication that is used to manage their neuropathies.
Examples of malignancy associated neuropathies include:
- Neuropathy due to chemotherapy side effects
- Neuropathy due to monoclonal gammopathies
- Neuropathy due to tumor compression
- Neuropathy due to tumor infiltration
| NEUROPATHIES ASSOCIATED WITH MALIGNANCY | |||
| NEUROPATHY | SIGNS/ SYMPTOMS | TREATMENT | PROGNOSIS |
| Chemotherapy side effects neuropathy | Tingling sensation, burning sensation, stabbing pain, and complete numbness. | Steroids, antiseizure drugs, opioids, antidepressants | Good prognosis since once the chemotherapy is withdrawn then symptoms disappear. |
| Monoclonal gammopathies neuropathy | Sensory ataxia, tingling sensation, and numbness. | plasmapheresis | Fair since the side effects occurs ten years later and the mortality rate is low |
| Tumor compression neuropathy | Loss of function, numbness, weakness, loss of balance | Removal of the tumor. | |
| Tumor infiltration neuropathy |
Malignancy associated neuropathies.
What are Neuropathies Caused by Physical Forces?
Neuropathies caused by physical forces are neuron damage due to physical disturbances.
Neuropathies caused by physical forces include:
- Neuropathy due to avulsion
- Carpal tunnel syndrome
- Neuropathy due to compression
- Neuropathy due to laceration
- Ulnar nerve entrapment
- Compression neuropathy of the radial nerve
| NEUROPATHIES CAUSED BY PHYSICAL FORCES | |||
| NEUROPATHY | SIGNS/ SYMPTOMS | TREATMENT | PROGNOSIS |
| Neuropathy due to avulsion | Weakness, inability to use certain muscles, total lack of movement, | Nerve transfer, | Fair if the repair is delayed and it can lead to total loss of function. |
| Carpal tunnel syndrome | Numbness, tingling, weakness, muscle damage, pain, | Wrist brace, positioning, reduce swelling, ergonomics,vitamin B6, median nerve gliding exercise. | Good as it can be manageable with treatment. |
| Neuropathy due to compression | Numbness, tingling, weakness, muscle damage, pain, | Reduce compression, NSAIDS, corticosteroids, | Good but may only cause permanent nerve damage and loss of function. |
| Neuropathy due to laceration | Inability to sense pain, numbness, tingling sensation, difficulty in walking | Suturing. | Fair since it has good recovery once surgery is done. |
| Ulnar nerve entrapment | Pain, numbness, tingling, loss of muscle mass, weakness | Occupation therapy, splints, pain medication | Fair since one can have full recovery. |
| Neuropathy due to radial nerve compression | Weakness of the fingers, loss of finger coordination, inability to straighten and stretch the arm. | Nsaids, corticosteroids, supportive splint, physical therapy. | Good since one recovers after treatment |
Table showing neuropathies caused by physical forces.
What are Inherited Peripheral Neuropathies?
Inherited peripheral neuropathies are diseases of the nerves that one inherits from the parents.
Examples of Inherited peripheral neuropathies include:
- Charcot-Marie-tooth disease
- Neuropathy due to familial amyloidosis
- Hereditary motor neuropathies
- Hereditary sensory neuropathies
- Neuropathy due to Inherited metabolic diseases
What is Charcot-Marie-Tooth Disease?
Charcot-Marie-tooth disease is an inherited neurological disorder that affects both sensory and motor nerves.
What is the Pathology of Charcot-Marie-Tooth Disease?
The pathology of Charcot-Marie-tooth disease is the study of the motor and the sensory disease of the nerves.
-Etiology: The cause of charcot-marie-tooth disease is gene mutation.
-Genes involved: GARS, PMP22, ATP1A1, PRPS1 genes.
-Pathogenesis: The sequence of events that lead to charcot-marie-tooth disease is increased duplication of the genes involved in the myelin sheath leading to the abnormality of the affected areas.
-Morphology: NA.
-Histology: NA.
How does Charcot-Marie-Tooth Disease Present?
Patients with charcot-marie-tooth disease typically are women present at the age range of childhood to 20 years. The symptoms, features, and clinical findings associated with charcot-marie-tooth include tripping and falling a lot, stork leg appearance due to muscle loss, bony abnormalities- high arch and hammertoes, sharp pain of the limbs, emaciation,
How is Charcot-Marie-Tooth Disease Diagnosed?
Charcot-marie-tooth disease is diagnosed by physical examination, history taking, neurology, and electrocardiography.
How is Charcot-Marie-Tooth Disease Treated?
Charcot-marie-tooth disease is treated by correction of the deformities, tendon lengthening, occupation therapy, and also pain medication.
What is the Prognosis of Charcot-Marie-Tooth Disease?
The prognosis of charcot-marie-tooth disease is good since one with the disease can live a normal life with supportive therapy.
What is Neuropathy due to Familial Amyloidosis?
Neuropathy due to familial amyloidosis is an autosomal dominant disease in which the nerves are damaged by the presence of fibrin and proteins.
What is the Pathology of Neuropathy due to Familial Amyloidosis?
The pathology of neuropathy due to familial amyloidosis is:
-Etiology: The cause of neuropathy due to familial amyloidosis is the mutation of the TTR gene which causes the accumulation of fibrin on the nerves.
-Genes involved: TTR genes.
-Pathogenesis: The sequence of events that lead to neuropathy due to familial amyloidosis are accumulation and the deposition of the fibrin and the proteins on the nerves.
-Morphology: The morphology associated with neuropathy due to familial amyloidosis shows plenty of amyloid fibrils.
-Histology: NA.
How does Neuropathy due to Familial Amyloidosis Present?
Patients with neuropathy due to familial amyloidosis are typically females present at the age range of 30-40years. The symptoms, features, and clinical findings associated with neuropathy due to familial amyloidosis include numbness and burning sensation of the feet and arms, paresthesia, balance challenges, vomiting, nausea, and diarrhea.
How is Neuropathy due to Familial Amyloidosis Diagnosed?
Neuropathy due to familial amyloidosis is diagnosed by blood tests, urinalysis, physical examination, history taking, electromyography, skin and muscle biopsy.
How is Neuropathy due to Familial Amyloidosis Treated?
Neuropathy due to familial amyloidosis is treated with antiseizure, antidepressant, and pain medication.
What is the Prognosis of Neuropathy due to Familial Amyloidosis?
The prognosis of neuropathy due to familial amyloidosis is fair with a survival rate of 80%.
What is Hereditary Motor Neuropathies?
Hereditary motor neuropathies is a progressive disorder of the nervous system that mostly affects the spinal cord.
What is the Pathology of Hereditary Motor Neuropathies?
The pathology of hereditary motor neuropathies is:
-Etiology: The cause of hereditary motor neuropathies is the malfunction of the heat shock proteins known as beta-1 and beta-8 triggered by mutation of the HSPB1 and HSPB8 genes.
-Genes involved: HSPB1, HSPB8 genes.
-Pathogenesis: The sequence of events that lead to hereditary motor neuropathies are atypical degeneration and development of the neural tissues.
-Morphology: NA.
-Histology: The histology associated with hereditary motor neuropathies shows onion bulb formation on the affected nerve.
How does Hereditary Motor Neuropathies Present?
Patients with hereditary motor neuropathies typically are males present at the age range of childhood and young adulthood. The symptoms, features, and clinical findings associated with hereditary motor neuropathies include fatigue, pain, lack of balance, loss of sensation, loss of hearing and sight, loss of reflexes.
How is Hereditary Motor Neuropathies Diagnosed?
Hereditary motor neuropathies are diagnosed with physical exam and history taking, electromyography, and genetic testing.
How is Hereditary Motor Neuropathies Treated?
Hereditary motor neuropathies are treated by physical therapy, use of braces, orthopedic surgery.
What is the Prognosis of Hereditary Motor Neuropathies?
The prognosis of hereditary motor neuropathies is fair since the symptoms occur later in life after the disease has progressed.
What is Hereditary Sensory Neuropathies?
Hereditary Sensory Neuropathies is a condition that emerges during childhood in which abnormalities of the nerves cause reduced ability to feel pain, reduced ability to sense cold or hot surfaces.
What is the Pathology of Hereditary Sensory Neuropathy?
The pathology of hereditary sensory neuropathy is:
-Etiology: The cause of hereditary sensory neuropathy is the mutation of genes.
-Genes involved: SPTLC1 gene.
-Pathogenesis: The sequence of events that lead to hereditary sensory neuropathy is the mutation of the genes responsible to finalize the sensory transmission is affected and the communication between the brain and the nerves is affected hence one is not able to sense a cold or hot surface despite the brain being aware of it.
-Morphology: NA.
-Histology: NA.
How does Hereditary Sensory Neuropathy Present?
Patients with hereditary sensory neuropathy typically are male present at the age range of 37 years. The symptoms, features, and clinical findings associated with hereditary sensory neuropathy include paresthesia, reduced ability to feel cold or hot, reduced ability to sense pain.
How is Hereditary Sensory Neuropathy Diagnosed?
Hereditary sensory neuropathy is diagnosed by nerve biopsies, nerve conduction study, physical examination, blood tests.
How is Hereditary Sensory Neuropathy Treated?
Hereditary sensory neuropathy is treated with orthopedic surgery, pain medication, physical therapy.
What is the Prognosis of Hereditary Sensory Neuropathy?
The prognosis of hereditary sensory neuropathy is good with a normal life expectancy period.
What is Neuropathy due to Inherited Metabolic Diseases?
Neuropathy due to Inherited metabolic diseases is the disease of the nervous system caused by a chemical disruption in the body that leads to the alteration of the structure and the function of the myelin and the axons.
What is the Pathology of Neuropathy due to Inherited Metabolic Diseases?
The pathology of neuropathy due to inherited metabolic diseases is:
-Etiology: The cause of neuropathy due to inherited metabolic diseases is inherited disorders such as porphyria, uremia, and diabetes.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to neuropathy due to inherited metabolic diseases is not well known but various factors can lead to it. They include diabetic polyneuropathy.
-Morphology: NA.
-Histology: NA.
How does Neuropathy due to Inherited Metabolic Diseases Present?
Patients with neuropathy due to inherited metabolic disorders typically are males and females present at the age range of 60-70 years. The symptoms, features, and clinical findings associated with neuropathy due to inherited metabolic diseases include numbness, difficulty in walking, lethargy, poor appetite,
How is Neuropathy due to Inherited Metabolic Diseases Diagnosed?
Neuropathy due to inherited metabolic diseases is diagnosed through history taking, physical examination, sensory and motor testing, urinalysis, thyroid function tests.
How is Neuropathy due to Inherited Metabolic Disorders Treated?
Neuropathy due to inherited metabolic diseases is treated and management of the underlying metabolic disorder is the main approach.
What is the Prognosis of Neuropathy due to Inherited Metabolic Diseases?
The prognosis of neuropathy due to inherited metabolic diseases is good depending on how well you will manage the underlying condition.
What are Diseases of the Neuromuscular Junction?
Diseases of the neuromuscular junction are conditions that occur in the neuromuscular junction causing poor communication between the muscles and the nerves.
Examples of diseases of the neuromuscular junction include:
- Myasthenia gravis
- Lambert-Eaton myasthenic syndrome
- Congenital myasthenic syndrome
- Toxin mediated diseases of the neuromuscular junction
What is Myasthenia Gravis?
Myasthenia gravis is an autoimmune disorder that leads to blockade at the neuromuscular junction causing antibodies to form against acetylcholine receptors causing muscle weakness.
What is the Pathology of Myasthenia Gravis?
The pathology of myasthenia gravis is:
-Etiology: The cause of myasthenia gravis is idiopathic but may be induced by drugs,
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to myasthenia gravis antibodies inhibit acetylcholine binding leading to muscle weakness.
-Morphology: NA.
-Histology: NA.
How does Myasthenia Gravis Present?
Patients with myasthenia gravis, typically female, are more present at the age range of 28 years in females and 42 years in males. The symptoms, features, and clinical findings associated with myasthenia gravis include painless specific muscle weakness, droopy eyelids, double vision, slurred speech, difficulty in swallowing, sad-looking facial appearance.
How is Myasthenia Gravis Diagnosed?
Myasthenia gravis is diagnosed with a history and physical exam, antibody testing, rheumatoid factor testing, radiography, CT scan, MRI.
How is Myasthenia Gravis Treated?
Myasthenia gravis is treated by a combination of cholinesterase inhibitors, immunosuppressors, plasmapheresis, immunotherapy, and supportive care.
What is the Prognosis of Myasthenia Gravis?
The prognosis of myasthenia gravis is good since the drugs used to reduce the mortality rate to 2-3%
What is Lambert-Eaton Myasthenic Syndrome?
Lambert-Eaton myasthenic syndrome is an autoimmune disease of the neuromuscular junction caused by antibodies that block the presynaptic release of acetylcholine.
What is the Pathology of Lambert-Eaton Myasthenic Syndrome?
The pathology of Lambert-Eaton myasthenic syndrome is:
-Etiology: The cause of lambert-eaton myasthenic syndrome is the presence of small cell lung cancer, autoimmune diseases,
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to lambert-eaton myasthenic syndrome antibodies attack the voltage-gated calcium channels causing insufficient depolarization which leads to a low release of acetylcholine.
-Morphology: NA.
-Histology: NA.
How does Lambert-Eaton Myasthenic Syndrome Present?
Patients with Lambert-Eaton myasthenic syndrome typically are both male and present at the age range of 30- 60 years. The symptoms, features, and clinical findings associated with lambert-eaton myasthenic syndrome include proximal muscle weakness, depressed tendon reflexes, post-tetanic potentiation, and autonomic changes.
How is Lambert-Eaton Myasthenic SyndromeDiagnosed?
Lambert-Eaton myasthenic syndrome is diagnosed by electromyography, physical examination, complete blood count, repetitive nerve stimulation.
How is Lambert-Eaton Myasthenic Syndrome Treated?
Lambert-Eaton myasthenic syndrome is treated by treating the underlying malignancy, avoiding drugs that increase muscle weakness.
What is the Prognosis of Lambert-Eaton Myasthenic Syndrome?
The prognosis of the lambert-eaton myasthenic syndrome is hard to determine since the type of cancer one presents with is the main key.
What is Congenital Myasthenic Syndrome?
Congenital myasthenic syndrome is a group of conditions characterized by muscle weakness, poor muscle tone, difficulty in breathing and eye weakness that worsens upon physical exertion.
What is the Pathology of Congenital Myasthenic Syndrome?
The pathology of congenital myasthenic syndrome is:
-Etiology: The cause of congenital myasthenic syndrome is a genetic mutation.
-Genes involved: CHRENE, RAPSN CHAT COLQ, DOK7.
-Pathogenesis: The sequence of events that lead to congenital myasthenic syndrome are abnormal signaling transmission of the motor endplate.
-Morphology: NA.
-Histology: NA.
How does Congenital Myasthenic Syndrome Present?
Patients with congenital myasthenic syndromes are typically female at an age range of from childhood to 6 years. The symptoms, features, and clinical findings associated with congenital myasthenia syndrome include the inability to walk.
How is Congenital Myasthenia Syndrome Diagnosed?
Congenital myasthenia syndrome is diagnosed with serum levels.
How is Congenital Myasthenia Syndrome Treated?
Congenital myasthenia syndrome is treated by management of respiratory distress, prevention of malnutrition and infection, beta 2 agonists.
What is the Prognosis of Congenital Myasthenia Syndrome?
The prognosis of congenital myasthenia syndrome is fair if there is no involvement of the respiratory system.
What are Toxin Mediated Diseases of the Neuromuscular Junction?
Toxin mediated diseases of the neuromuscular junction are diseases caused by toxins at the neuromuscular junction affecting the activities that occur there. One example is Botulism
What is the Pathology of Toxin Mediated Disease of the Neuromuscular Junction?
The pathology of toxin-mediated diseases of the neuromuscular junction is the study of the toxins affecting the neuromuscular junction.
-Etiology: The cause of botulism is neurotoxins from food, wound.
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to botulism, neurotoxins act on the neuromuscular junction causing blockade of the acetylcholine release leading to the abnormal function of the junction.
-Morphology: NA.
-Histology: NA.
How does Botulism Present?
Patients with botulism typically are both male and female present at the age range of 2-8 monthsThe symptoms, features, and clinical findings associated with botulism include nausea, vomiting, blurred vision, paralysis.
How is Botulism Diagnosed?
Botulism is diagnosed by stool specimen blood tests, physical examination and history taking.
How is Botulism Treated?
Botulism is treated according to the presenting symptoms, ventilation, surgery.
What is the Prognosis of Botulism?
The prognosis of botulism is poor with a mortality of 50%
What are Peripheral Nerve Sheath Tumors?
Peripheral nerve sheath tumors are benign tumors of the peripheral nerve sheath.
Examples of peripheral nerve sheath tumors include:
- Schwannomas
- Neurofibromas
- Malignant peripheral nerve sheath tumors
- Neurofibromatosis
What are Schwannomas?
Schwannomas are benign tumors of the tissue that covers the nerve cells. Also defined as the benign tumors of Schwann cell lineage. Antoni A, Antoni B, verocay bodies.
What is the Pathology of Schwannomas?
The pathology of schwannomas is:
-Etiology: The cause of schwannomas is not fully known.
-Genes involved: SMARCB1, LZTR1 genes.
-Pathogenesis: The sequence of events that lead to schwannomas loss of function of the merlin caused by genetic alteration or its inactivation leading to growth of schwannomas.
-Morphology: The morphology associated with schwannomas shows well-circumscribed masses on the nerve.
-Histology: The histology associated with schwannomas shows the presence of Antoni A, Antoni B, and verocay bodies.
How does Schwannomas Present?
Patients with schwannomas typically both male and female present at the age range of 30-60 years. The symptoms, features, and clinical findings associated with schwannomas include difficulty in walking, loss of balance, headache, vertigo, dizziness, tinnitus, facial numbness, visual disturbances.
How is Schwannomas Diagnosed?
Schwannomas are diagnosed by CT scan, clinical examination, MRI, and biopsy.
How is Schwannomas Treated?
Schwannomas are treated by surgical resection.
What is the Prognosis of Schwannomas?
The prognosis of schwannomas is good with no occurrence after surgical resection.
What are Neurofibromas?
Neurofibromas are benign peripheral nerve sheath tumors and can be superficial cutaneous, diffuse, or plexiform.
What is the Pathology of Neurofibroma?
The pathology of neurofibromas is:
-Etiology: The cause of neurofibromas is a genetic mutation.
-Genes involved: NF1 gene
-Pathogenesis: The sequence of events that lead to neurofibromas is non-myelinating Schwann cells that grow on the peripheral nerves and can increase in size hence becoming a neurofibroma.
-Morphology: The morphology associated with neurofibroma shows soft pedunculated or sessile papules.
-Histology: The histology associated with neurofibroma shows a non-encapsulated lesion on the dermis, mast cells, fibroplasia, and mucin deposition.
How does Neurofibroma Present?
Patients with neurofibroma typically both males and females present at the age range of 20-40 years. The symptoms, features, and clinical findings associated with neurofibroma include flat, light brown spots on the skin, bone deformities, bumps on or under the skin, Lisch nodules.
How is Neurofibroma Diagnosed?
Neurofibroma is diagnosed by CT scan, physical examination, MRI, PET scan, and biopsy.
How is Neurofibroma Treated?
Neurofibroma is treated by close monitoring, and surgery when indicated.
What is the Prognosis of Neurofibromas?
The prognosis of neurofibroma is good.
What are Malignant Peripheral Nerve Sheath Tumors?
Malignant peripheral nerve sheath tumors are cancerous tumors of the nerve sheath.
What is the Pathology of Malignant Peripheral Nerve Sheath Tumors?
The pathology of malignant peripheral nerve sheath tumors is:
-Etiology: The cause of malignant peripheral nerve sheath tumors is not clear but the risk factors are radiation therapy, nerve tumors,
-Genes involved: NA.
-Pathogenesis: The sequence of events that lead to malignant peripheral nerve sheath tumors, is mediated when the cells of the nerve sheath show differentiation and new cells arise either from the existing tumor or from the peripheral nerves.
-Morphology: The morphology associated with malignant peripheral nerve sheath tumors shows mitotically active cells with monomorphic spindle cells, scant cytoplasm, and fine chromatin.
-Histology: the histology associated with malignant peripheral nerve sheath tumors shows marbled appearance, uniform cellularity, and heterogenous differentiation.
How does Malignant Peripheral Nerve Sheath Tumors Present?
Patients with malignant peripheral nerve sheath tumors typically present at the age range of 20-60 years. The symptoms, features, and clinical findings associated with malignant peripheral nerve sheath tumors include pain in the affected area, weakness while moving, growing lump under the tissue.
How is Malignant Peripheral Nerve Sheath Tumor Diagnosed?
Malignant peripheral nerve sheath tumor is diagnosed by neurological examination, imaging tests, biopsy.
How is Malignant Peripheral Nerve Sheath Tumor Treated?
Malignant peripheral nerve sheath tumors are treated by surgery, radiation therapy, chemotherapy, pain medication, rehabilitation.
What is the Prognosis of Malignant Peripheral Sheath Tumors?
The prognosis of malignant peripheral sheath tumors is fair with a survival rate of 64%.
What is Neurofibromatosis?
Neurofibromatosis is a benign nerve tumor that forms bumps under the skin and it is known to develop anywhere in the body.
Two types of neurofibromatosis include:
Type 1 neurofibromatosis:
Type 2 neurofibromatosis:
What is Neurofibromatosis Type 1?
Neurofibromatosis Type 1 is an inherited condition that causes benign tumors to grow along the nerves characterized by the café au lait spots, lisch nodules.
What is the Pathology of Neurofibromatosis Type 1?
The pathology of neurofibromatosis type 1 is:
-Etiology: The cause of neurofibromatosis type 1 is genetic mutation.
-Genes involved: NF1 tumor-suppressor gene.
-Pathogenesis: The sequence of events that lead to neurofibromatosis type 1: mutation of the NF-1 gene causes loss of production of the protein that is responsible for increasing the chances of growth of tumors.
-Morphology: The morphology associated with neurofibromatosis type 1 shows large volumes of cortical and subcortical masses.
-Histology: The histology associated with neurofibromatosis type 1 shows mast cells, macrophages, endothelial cells, pericytes, and perineural cells.
How does Neurofibromatosis Type 1 Present?
Patients with neurofibromatosis type 1 typically are both male and female present at age range of 10 years, childhood. The symptoms, features, and clinical findings associated with neurofibromatosis type 1 include café au lait, freckles on the armpits and groin, Lisch nodules, skeletal abnormalities e.g. osteopenia, and cardiovascular diseases,
How is Neurofibromatosis Type 1 Diagnosed?
Neurofibromatosis type 1 is diagnosed history taking, physical examination, CT scan, MRI, complete blood count, and biopsy.
How is Neurofibromatosis Type 1 Treated?
Neurofibromatosis type 1 is treated by mainly controlling the underlying symptoms. Other options include surgery, and pain medication.
What is the Prognosis of Neurofibromatosis Type 1?
The prognosis of neurofibromatosis is fair.
What is Neurofibromatosis Type 2?
Neurofibromatosis type 2 is a condition of the nerves where slow-growing tumors develop in both ears leading to bilateral hearing loss due to the presence of schwannomas on the 8th cranial nerve.
What is the Pathology of Neurofibromatosis Type 2?
The pathology of neurofibromatosis type 2 is:
-Etiology: The cause of neurofibromatosis type 2 is a genetic mutation.
-Genes involved: NF-2 gene.
-Pathogenesis: The sequence of events that lead to neurofibromatosis type 2 not clearly understood
-Morphology: The morphology associated with neurofibromatosis type 2 shows well-circumscribed tumors.
-Histology: The histology associated with neurofibromatosis type 2 shows Antoni A and Antoni B structures.
How does Neurofibromatosis Type 2 Present?
Patients with neurofibromatosis type 2 typically are female and males present at the age range of 18-24 years. The symptoms, features, and clinical findings associated with neurofibromatosis type 2 include gradual hearing loss, poor balance, headaches, tinnitus.
How is Neurofibromatosis Type 2 Diagnosed?
Neurofibromatosis type 2 is diagnosed history taking and physical examination. MRI, CT scan, biopsy, hearing tests, vision tests, and genetic testing.
How is Neurofibromatosis Type 2 Treated?
Neurofibromatosis type 2 is treated depending on the size and the location. It can be surgery, radiation, auditory brainstem implant, and chemotherapy.
What is the Prognosis of Neurofibromatosis Type 2?
The prognosis of neurofibromatosis type 2 is poor due to its high mortality rate at a young age.