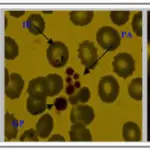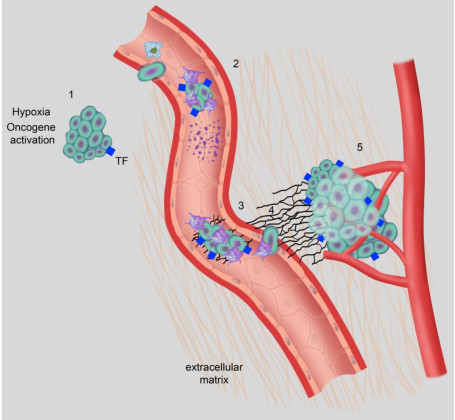
An overview of important interactions between hemostasis and breast cancer. (1) Hypoxia and oncogenic activation increase tissue factor expression, which increases urokinase-type plasminogen activator (uPA) expression and transcription in tumor cells, facilitating intravasation. (2) Tumor cell-induced platelet aggregation leads to platelet activation. Activated platelets subsequently shed microparticles, release various growth mediators, and protect tumor cells from immune-mediated destruction in circulation. (3) Platelets help in adhesion of tumor cells through p-selectin and integrins, aiding in extravasation from the circulation. (4) Thrombin generated through TF-FVIIa-Xa pathway upregulates growth-related oncogene-alpha (GRO-a) and leads to secretion of matrix metalloproteinase (MMP) and angiogenic molecules. (5) Tissue factor, platelets, thrombin, and the plasminogen system enhance tumor growth, invasion, and angiogenesis, leading to clonogenic accumulation of tumor cells outside breast tissue. FVIIa, activated factor VII; TF, tissue factor. Platelets, coagulation and fibrinolysis in breast cancer progression. Lal I, Dittus K, Holmes CE - Breast cancer research : BCR (2013). Not Altered. CC.
Disorders of secondary hemostasis are caused by failure of fibrin clot formation due to deficiency of one or more coagulation factors. Disorders of secondary hemostasis can be due to factor abnormalities.
Examples of disorders of secondary hemostasis include:
- Hemophilia A
- Hemophilia B
- Coagulation factor inhibitor
- Von willebrand disease
- Disorder of hemostasis due to vitamin K deficiency
- Liver failure related disorder of hemostasis
- Large volume transfusion related disorder of hemostasis