Growth adaptations are cellular changes made in response to environmental stressors that disrupt homeostasis (the state of relative equilibrium maintained by living systems). Growth adaptations include hyperplasia, hypertrophy, atrophy, metaplasia, dysplasia, aplasia, and hypoplasia. If the cells are past their adaptability to respond to a change in the environment such as lack of nutrients, oxygen deprivation, or toxic chemical exposure cell injury may occur. Cell injury can be reversible or irreversible. Progressive cell injury may lead to cell death.
WHAT ARE GROWTH ADAPTATIONS?
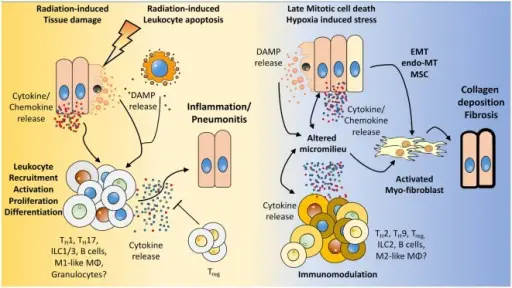
How the microenvironment shapes the immune response and vice versa. We hypothesize that radiation induces damage to tissue resident cells, e.g., endothelial and epithelial lung cells, mesenchymal stem cells (MSC) as well as in resident immune cells. The resulting tissue damage can initiate stress responses or cell death with subsequent release of cytokines/chemokines and damage-associated molecular patterns (DAMPs). This initial damage response leads to the recruitment and activation of diverse immune cells to the lung, among them lymphocytes. Further activation, proliferation of these cells, and secretion of cytokines shape the pulmonary micromilieu toward inflammation and—if this response is too excessive—to the development of severe pneumonitis. Late chronic mitotic cell death and subsequent tissue hypoxia lead to the release of DAMPs and cytokines/chemokines from resident cells thereby altering the micromilieu in the lung. These environmental changes impact on the immune cells present in the lung tissue and promote an altered cytokine release of immune cells. Finally, epithelial-mesenchymal-transition, endothelial-mesenchymal-transition, mesenchymal stem cell differentiation, and the altered environment contribute to the induction of activated myofibroblasts, collagen deposition, and fibrosis. The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung. Frontiers in Immunology. Not Altered. CC.