Cell death is the event of a biological cell ceasing to carry out its functions and it occurs when cultures become overcrowded. Morphologically, cell death can be classified into four different forms: apoptosis, autophagy, necrosis, and entosis. This may be the result of the natural process of old cells dying and being replaced by new ones, or of the disease, localized injury, and the death of the organism of which the cells are part.
What is Apoptosis?
Apoptosis is the process of programmed cell death and it’s used during early development to eliminate unwanted cells; for example, those between the fingers of a developing hand. In adults, apoptosis plays a role in preventing cancer and is used to rid the body of cells that have been damaged beyond repair.
Apoptosis results from numerous stimuli, including ischemia, hypoxia, genotoxic damage, viral infection, microtubule disruption, ER damage, hypoglycemia, ROS, mitochondrial damage, death receptor activation, growth factor withdrawal, anoikis (detachment from ECM). Also, apoptosis can result from the exposure to certain drugs and chemicals, immune reactions, infectious agents, high temperature, radiation, and various disease states.
The mechanisms of apoptosis include the intrinsic pathway, and the extrinsic pathway. These two pathways result in caspase activation in the mitochondrial pathway as well as the death receptor pathway.
What are the Causes of Apoptosis?
The causes of apoptosis are situational, and include physiologic issues that may lead to apoptosis (physiologic apoptosis), or pathologic issues that may lead to apoptosis (pathologic apoptosis).
What is Physiologic Apoptosis?
Physiologic apoptosis is a process essential for normal health and, since the process is mediated by specific proteins encoded in the host’s genome, it’s also a programmed cell death. It occurs normally for maintenance of tissue homeostasis and plays an important role in morphogenesis, embryogenesis, and tissue growth.
Examples of issues that promote physiologic apoptosis include:
- Destruction of cells throughout embryogenesis
- Hormone induce involution of tissues
- Cells lost in cell populations that are proliferating
- Elimination of harmful cells
- Elimination of self-reactive lymphocytes
- Elimination of cells after they have served their purpose
What is Pathologic Apoptosis?
Pathologic apoptosis is a sign of disease and damage and it’s involved in different pathological processes such as malignancy, infectious diseases and autoimmune disorders. Examples of issues that promote pathologic apoptosis include:
- DNA damage
- Misfolded protein build up
- Infection related Atrophy
What are the Morphologic Features of Apoptosis?
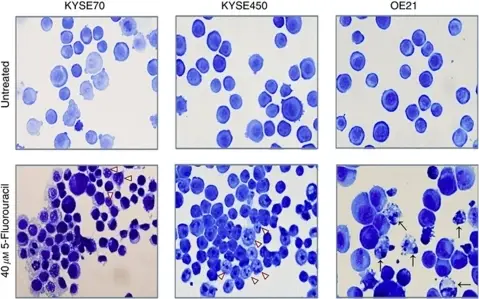
The morphologic features of apoptosis include: cell shrinkage, chromatin condensation, formation of apoptotic bodies, cytoplasmic blebs, and phagocytosis of apoptotic bodies and cytoplasmic blebs.
Cell shrinkage is a ubiquitous characteristic of programmed cell death. It is observed in all examples of apoptosis, independent of the death stimulus. Cell shrinkage, also known as apoptotic volume decrease (AVD), plays an important role during cell death regulating in particular the activity of apoptotic nucleases and the activation of caspases.
Chromatin condensation is one of the most important criteria, paralleled by DNA fragmentation, which are microscopic features used to identify apoptotic cells. There is a rapid degradation of the nuclease-hypersensitive euchromatin that leads to collapse of the nucleus and aggregation of heterochromatin to produce the appearance of condensed apoptotic chromatin.
Apoptotic bodies are one of the morphologic phenomena that can be observed during the process of apoptosis and they are formed through a process termed apoptotic cell disassembly characterized by a series of tightly regulated morphological steps including plasma membrane blebbing, apoptotic membrane protrusion formation and fragmentation into apoptotic bodies. These membrane-surrounded fragments are cleared by phagocytosis without triggering an inflammatory response. Apoptotic bodies are the reason for the Greek term “apoptosis”, which means falling away.
Cytoplasmic blebs are protrusions of the cell membrane and they are the result of actomyosin contractions of the cortex, which cause either transient detachment of the cell membrane from the actin cortex or a rupture in the actin cortex. Then, cytosol streams out of the cell body and inflates the newly formed bleb.
Phagocytosis is the cellular engulfment of large particles (>0.5 μm), including microorganisms, foreign substances, and apoptotic cells. Clearance of apoptotic cells by phagocytic cells plays a significant role in the resolution of inflammation, protecting tissue from harmful exposure to the inflammatory and immunogenic contents of dying cells. Apoptosis induces cell surface changes that are important for recognition and engulfment of cells by phagocytes and these changes include alterations in surface sugars, externalization of phosphatidylserine and qualitative changes in the adhesion molecule ICAM-3.
What is the Intrinsic Pathway of Apoptosis?
The intrinsic pathway is used to eliminate cells in response to genotoxic damage, mitochondrial damage, absence of growth factors, loss of substrate adhesion, etc. Intrinsic apoptosis is triggered from within the cell and it involves the release of cytochrome-C from the mitochondria. Cytochrome-C is released into the cytoplasm to initiate apoptosis, and results in channel formationby 2 members of a large family of proteins, called Bax & Bak. These activated proteins lead to mitochondrial outer membrane permeabilization. This activates caspases through the release of proteins such as cytochrome-C or SMAC and OMI from the mitochondria and drive further caspase activation in the case of cytochrome-C. Cytochrome-C binds an adaptor molecule Apaf that further activates caspases, they cleave hundreds of different proteins bringing about rapid apoptotic cell death. SMAC and OMI, on the other hand, inhibits a caspase inhibitor called XIAP, and the net effect is once mitochondrial permeabilization has occurred, also known as MOMP, the cell dies within a matter of minutes to hours.
What is the Extrinsic Pathway of Apoptosis?
The extrinsic pathway is responsible for elimination of cells during development, termination of immune responses, and for immunosurveillance. Extrinsic apoptosis is triggered from outside the cell and it is initiated by activation of tumor necrosis factor (TNF) receptor family of “cell death receptors”- such as the Fas receptor. Extrinsic apoptosis uses caspases 8 and 10 for initiation of apoptosis. Killer lymphocytes express Fas ligand (FasL) on their cell surface. A killer lymphocyte binds to target B cell via the Fas Death Receptor. FasL and Fas Death Receptor binding recruits FADD adaptor molecules (Fas-Associated protein with Death Domain) via binding between the receptor “death domain” and adaptor death domain. Pro-caspases aggregate by binding to the “death effector domain” of the adaptor forming the Death-Inducing Signaling Complex (DISC). Then, procaspases are cleaved and lead to apoptosis.
What is the Execution Phase of Apoptosis?
The execution phase of apoptosis is where the intrinsic and extrinsic pathways converge which leads to a caspase activation. Caspases are a family of cysteine proteases that are the primary effectors of apoptosis that proteolytically dismantle most cellular structures.
What is Necroptosis?
Necroptosis is a programmed form of necrosis, or inflammatory cell death. Necrosis is cell death due to accident or acute injury and is not a controlled process.
What is Pyroptosis?
Pyroptosis is a highly inflammatory form of lytic programmed cell death that occurs most frequently upon infection with intracellular pathogens and is likely to form part of the antimicrobial response. In contrast to apoptosis and necrosis, pyroptosis requires the function of the enzyme caspase-1.
How are Dead Cells Removed?
Removal of dead cells is happening by phagocytes such as macrophages and dendritic cells. Clearance of normal dead cells occurs silently in immune tolerance. Exogenous or mutated antigens of malignant or infected cells can initiate adaptive immunity, thereby inducing immunogenicity by adjuvant signals.
What is Autophagy?
Autophagy is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through lysosomal-dependent regulated mechanisms. Autophagy involves the formation of a double-membrane vesicle that encapsulates cytoplasm around targeted material for degradation that fuses with lysosomes for degradation.
Chaperone mediated autophagy is a proteolytic systems that contributes to degradation of intracellular proteins in lysosomes. Chaperone mediated autophagy substrate proteins are selectively targeted by ubiquitins and translocated into the lysosomal lumen through the coordinated action of chaperones located at both sides of the membrane and a dedicated protein translocation complex.
What are Intracellular Accumulations?
Intracellular accumulations are endogenous by-products and exogenous substances accumulated by injured cells. Examples of intracellular accumulations include:
- Normal cellular constituent accumulated in excess, such as water, lipids, proteins, and carbohydrates
- Abnormal substances (exogenous, such as a mineral or products of infectious agents, or endogenous, such as a product of abnormal synthesis or metabolism)
- Pigments
Intracellular accumulations may occur due to metabolic abnormalities, genetic mutations, or exposure to an indigestible exogenous substance. Some of these accumulations are relatively harmless while others can have drastic effects on health and cognitive function which may include seizures or even death.
What Intracellular Accumulations are due to Lipids?
Intracellular accumulations due to lipids include may result in fatty changes. This is usually apparent in parenchymal cells such as the liver. Steatosis is the accumulation of lipid particles within parenchymal cells. Intracellular lipid accumulation can develop in many organs and tissues, but because the liver is so important to lipid metabolism, hepatic lipidosis is more common.
Atherosclerosis is hardening or thickening of the arteries caused by a buildup of plaque in the inner lining of an artery. Risk factors for atherosclerosis include high cholesterol levels, high triglyceride levels, high blood pressure, smoking, diabetes, obesity, and lack of physical activity.
Cholesterolosis is accumulation of lipid-containing foamy macrophages in the lamina propria of the gallbladder. Grossly this accumulation is seen as yellow mucosal flecks, linear streaks, or a meshlike network.
What Intracellular Accumulations are due to Proteins?
Intracellular accumulations due to proteins are due to the excessive accumulation of aminoacids or proteins that may have negative effects on homeostasis and health.
Examples of intracellular accumulations due to proteins include:
- Alpha-1-antitrypsin deficiency
- Phenylketonuria
What Intracellular Accumulations are due to Hyaline Change?
Intracellular accumulations due to hyaline change include the protein resorption vesicles in the apical cytoplasm of proximal renal tubular epithelial cells that is seen in protein-losing nephropathy. Russell bodies are another example of intracellular accumulations due to hyaline change.
What Intracellular Accumulations are due to Glycogen?
Intracellular accumulations due to glycogen include hepatocellular, clear vacuoles of glycogen in the cytoplasm of cells. They are irregularly shaped than the vacuoles of hepatic lipidosis. They may be seen in glycogen storage diseases.
What Intracellular Accumulations are due to Pigments?
Intracellular accumulations due to pigments include may be from exogenous or endogenous sources.
Exogenous pigments include:
- Carbon (anthracosis)
- Coal dust (pneumoconiosis)
- Tattoo ink (india ink, carbon)
- Ingested (argyria-silver, lead, carotenemia)
Endogenous pigments include:
- Lipofuscin
- Melanin
- Hemosiderin
What is Pathologic Calcification?
Pathologic calcification is the deposition of calcium salts, typically as phosphates or carbonates, in soft tissues (i.e., tissues that would not be calcified in a healthy state). If calcification is extensive, it appears grossly as chalky white deposits with a brittle or gritty texture. There are two different types of pathologic calcification: dystrophic calcification and metastatic calcification.
What is Dystrophic Calcification?
Dystrophic calcification is the calcification of dead tissue as part of the process of necrosis. Calcification is the gross lesion for which the myocardial and skeletal muscle necrosis of vitamin E or selenium deficiency in ruminants was named white muscle disease. Calcification is also prominent in other forms of necrosis (e.g., in the caseous necrosis of tuberculoid granulomas), in parasitic granulomas, and in necrotic fat or lipomas (benign neoplasms of adipocytes).
What is Metastatic Calcification?
Metastatic calcification is soft tissue calcification as the result of elevated serum calcium concentration. It targets the intima and tunica media of vessels, especially those in the lungs, pleura, endocardium, kidneys, and stomach. The primary defect is an imbalance in calcium and phosphate concentrations in the blood.
What is Cellular Aging?
Cellular aging is diminished function of cells and tissues from the molecular to the organismal level. DNA, especially telomeric DNA and metabolic pathway components affect the life span. The stem cell theory of aging states that critical shortening of telomeres results in a DNA damage response that activates p53 and leads to growth arrest, senescence, or apoptosis of the affected cell. The average cell loses telomere length at a rate of about 25 base pairs per year. When the telomere length decreases below a certain threshold, the cell undergoes aging. Telomeres may serve as a biological clock.