Cellular aging is diminished function of cells and tissues from the molecular to the organismal level. DNA, especially telomeric DNA and metabolic pathway components affect the life span. The stem cell theory of aging states that critical shortening of telomeres results in a DNA damage response that activates p53 and leads to growth arrest, senescence, or apoptosis of the affected cell. The average cell loses telomere length at a rate of about 25 base pairs per year. When the telomere length decreases below a certain threshold, the cell undergoes aging. Telomeres may serve as a biological clock.
What is Cellular Aging?
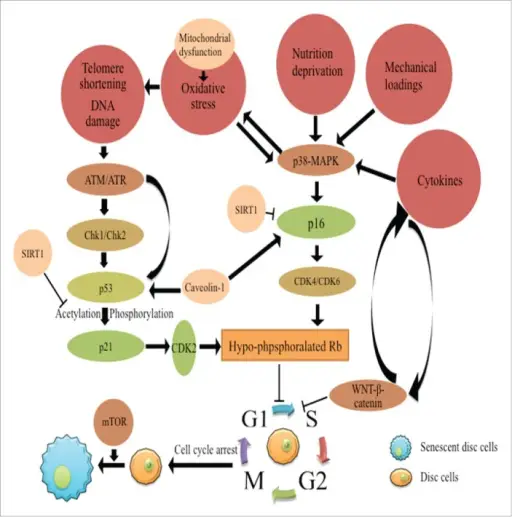
The molecular mechanism of disc cell senescence. The molecular mechanism underlying disc cell senescence includes 2 aspects, the arrest of cell cycle and the development of the senescent phenotype of disc cells. The p53-p21-Rb pathway and the p16-Rb pathway play major roles in the cell cycle arrest. Both pathways are activated by telomere shortening, DNA damage response or various stressful stimuli in the microenvironment of degenerative discs respectively. SIRT1 plays a protective role in disc cell senescence by suppressing p53 and p16. Caveoline-1 synergizes with p53 and p16 to accelerate disc cell senescence. The p38-MAPK pathway responds to various stimuli to activate the p53-p21-Rb and p16-Rb pathways. WNT-β-catenin pathway induces disc cell senescence. A positive-feedback loop of WNT signaling and cytokines enhances the pro-senescence effects of WNT-β-catenin pathway. Moreover, the mTOR pathway is required for cells to acquire the senescent phenotype. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways.
Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y - Cell cycle (Georgetown, Tex.) (2016). Not Altered. CC.