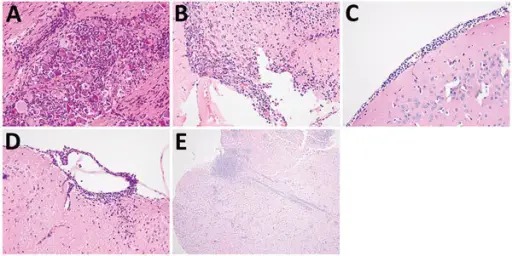
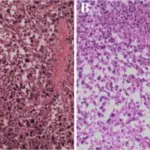
Central nervous system pathology comprises of all the neurological disorders that affect the structure or function of the brain or spinal cord, which together make up the central nervous system (CNS). Each disease has its own set of signs and symptoms. Findings associated with central nervous system pathology includes the inability to concentrate, loss of memory, decreased strength, diminished sensations, tremors, seizures, spasticity, paralysis and slurred speech.
How do Neurons React to Injury?
Neurons react to injury in many ways which include:
- Red neurons
- Subacute neuronal Injury
- Chronic neuronal Injury
- Axonal reaction
- Neuronal inclusions
What are Red Neurons?
A red neuron is a pathological finding in neurons which indicates an acute neuronal injury. It leads to eventual necrosis or apoptosis. The red color of red neurons is due to pyknosis and loss of Nissl bodies.
What is Subacute Neuronal Injury?
Subacute neuronal injury is neuronal death due to a progressive disease. The death is usually due to apoptosis and is associated with reactive gliosis. This includes cognitive defects, confusion, and impaired information processing.
What is Chronic Neuronal Injury?
Chronic neuronal injury refers to a series of biochemical pathways that leads to necrosis and apoptosis over a long period of time. This involves memory loss, motor dysfunction, and neurogenic inflammation. Chronic neuronal injury is mostly irreversible.
What is Axonal Reaction?
Axonal reaction are changes to axons that take place as a result of axonal damage. Axonal reaction most commonly indicates an axonal damage which follows damage to the cell body. The subsequent neuronal swelling is referred to as central chromatolysis.
What are Neuronal Inclusions?
Neuronal inclusions are basophilic round, oval or crescentic intracytoplasmic inclusions that stain pale blue with H&E staining. Occasionally small vacuoles are found within neuronal inclusions. Neuronal inclusions can be the size of the nucleus of a neuron.
How do Astrocytes React to Injury?
Astrocytes undergo a process known as gliosis in response to injury. Gliosis is characterized by hypertrophy, proliferation, and subsequent scar formation via the activation of signaling pathways.
How do Microglia React to Injury?
Microglia react to injury in many ways that include:
- Hypertrophy
- Scar
- Apoptosis
How do Oligodendrocytes React to Injury?
Oligodendrocytes react to injury with the hypertrophy and creating an inhibitory environment that prevents regeneration of axons.
How do Ependymal Cells React to Injury?
Ependymal cells react to injury by migrating to the site of injury and proliferating into neurons and glial cells in the adult spinal cord.
What is Cerebral Edema?
Cerebral edema is fluid buildup that may cause swelling of the brain. Cerebral edema is a life-threatening condition that causes fluid to accumulate in the brain. Cerebral edema can lead to an increase in intracranial pressure.Cerebral edema may be due to increased fluid leakage from blood vessels or cellular injury.
Types of cerebral edema include:
- Cytotoxic cerebral edema
- Interstitial cerebral edema
- Vasogenic cerebral edema
What is Cytotoxic Cerebral Edema?
Cytotoxic edema is fluid shift due to injury.
What is Interstitial Cerebral Edema?
Interstitial edema is fluid shift to intercellular spaces due to pressure of cerebrospinal fluid.
What is Vasogenic Cerebral Edema?
Vasogenic edema is fluid shift to intercellular spaces due to pressure.
What is Hydrocephalus?
Hydrocephalus is the accumulation of excess fluid in the ventricles within the brain. Hydrocephalus can result in an increase in size of the ventricles. As a result, the pressure on the brain increases. Hydrocephalus iis due to excessive accumulation of cerebrospinal fluid within the ventricular system.
What is Intracranial Pressure?
Intracranial pressure is the pressure exerted by fluids in the brain tissue inside the skull. This is mainly generated by cerebrospinal fluid. The normal value of CSF in a supine adult is 7-15 mmHg.
Raised intracranial pressure refers to the growing pressure inside the skull, above 20 mmHg. It leads to headache, vomiting, nausea, back pain, papilledema, ocular palsies and drowsiness.
One major complication of increased intracranial pressure is brain herniation.
What is Herniation of the Brain?
Herniation of the brain is the protrusion of brain tissue through openings in the rigid intracranial barriers. Herniation can cause displacement of the brain tissue through skull openings or past dural folds, due to increased intracranial pressure.
What are the Types of Brain Berniation?
Types of brain herniation include:
- Subfalcine (cingulate) herniation
- Tonsillar herniation
- Transtentorial (uncinate, mesial temporal) herniation
What are Malformations and Developmental Disorders of the Central Nervous System?
Malformations and developmental disorders of the central nervous system are birth defects of the brain and spinal cord that occur during the development of the foetus during pregnancy or in utero. Malformations and developmental disorders range from minor disorders to very serious abnormalities. They may be due to genetic and environmental causes.
Examples of malformations and developmental disorders of the central nervous system include:
- Neural tube defects
- Forebrain anomalies
- Posterior fossa anomalies
- Syringomyelia
- Hydromyelia
What are Neural Tube Defects?
Neural tube defects are birth defects of the spinal cord or brain that occur in the first month of pregnancy. Neural tube defects are caused by many factors such as folic acid deficiency, maternal insulin dependent diabetes, and antiepileptic medications.
Neural tube defects may include:
- Anencephaly
- Spina bifida
- Myelomeningocele
- Encephalocele
What is Anencephaly?
Anencephaly is the absence of a large portion of the brain, skull and scalp. Anencephaly occurs as a result of a neural tube defect during embryonic development, in which the neural tube fails to close at the rostral end.
What is Spina Bifida?
Spina bifida is a type of birth defect in which the spine and membranes around the spinal cord fail to close properly. The lower back is the most common site of spina bifida.
The two most common types of spina bifida are:
- Spina bifida occulta, which is the absence of bony spinous process.
- Spina bifida cystica which includes myelomeningocele (which contains neural elements along with meninges, and is most common), and meningocele (sac containing meninges bulges through the defect).
What is the Pathology of Spina Bifida?
Etiology: The causes of spina bifida includes genetic and environmental factors which include: folic acid deficiency, alcohol, antiepileptic medications, maternal diabetes mellitus, and obesity.
Genes involved: MTHFR is involved, which guides to the production of a protein that makes vitamin folate.
Pathogenesis: Spina bifida occurs when the vertebral neural arches fail to form in the first month of pregnancy. There is maldevelopment of the ectoderm, mesoderm, and neuroectodermal tissues, that causes the layers around the spinal cord and the spine fail to close.
Histology: The histology associated with spina bifida shows different germinal layers. Squamous epithelium or fibrous connective tissue can be present on the outer layer of myelomeningocele. Blood vessels and hyalinised connective tissue is also present.
How does Spina Bifida Present?
Patients with spina bifida typically constitute more females than males, and present during the first trimester. It can be detected on a 20th week ultrasound. The symptoms, features, and clinical findings associated with spina bifida include obvious bulging defect overlying the spine, and bladder issues.
How is Spina Bifida Diagnosed?
Spina bifida is diagnosed by physical examination or fetal ultrasound.
How is Spina Bifida Treated?
Spina bifida is treated symptomatically and with surgical procedures.
What is the prognosis of Spina Bifida?
The prognosis of spina bifida is poor for patients with complete paralysis or many complications such as hydrocephalus and congenital defects. However, those which do not experience such complications live their full lives.
What is Myelomeningocele?
Myelomeningocele is a type of spina bifida, in which the bones of the spine do not completely form. This results in an incomplete spinal canal through which the meninges and spinal cord protrude from the back.
What is the Pathology of Myelomeningocele?
Etiology: The causes of myelomeningocele include environmental, maternal and genetic factors.
Genes involved: Some genetic factors are involved such as the presence of chromosomal anomalies of trisomy 18 or 13. Family history of an affected twin or first-degree relative is also important.
Pathogenesis: The pathology of myelomeningocele is characterized by the incomplete closure of the spinal neural tube during the first month of pregnancy. Hence, the neural tissue and meninges protrude outwards. This exposed neural tissue is called a placode. The existence of a placode with the meningeal lining is a hallmark of myelomeningocele. Hence, the meninges along with the neural tissue protrude outwards. The spinal cord herniates outwards in the fluid filled sac.
Histology: The histology associated with myelomeningocele shows elements of all germ layers. The squamous epithelium or fibrous connective tissue covers the dome of the myelomeningocele that comprises of the neural placode. There may also be the presence of meninges, blood vessels, and hyalinized connective tissue.
How does Meningomyocele Present?
Patients with meningomyocele comprise of more females than males. Meningomyocele is usually obvious at birth.
How is Myelomeningocele Diagnosed?
Myelomeningocele is diagnosed by physical exam. Earlier diagnostic methods include ultrasound, and maternal serum alpha fetoprotein test.
How is Myelomeningocele Treated?
Myelomeningocele is treated by surgery.
What is the Prognosis of Myelomeningocele?
The prognosis of myelomeningocele is fairly good, and lifespan is generally normal.
What is Encephalocele?
Encephalocele is a sac like protrusion of the brain and covering membranes through an opening in the skull. Encephalocele happens when the neural tube fails to close during pregnancy. Encephalocele can lead to the formation of a groove on the skull.
What is the Pathology of Encephalocele?
The pathology of encephalocele is:
Etiology: The cause of encephaloceles is the failure of the neural tube to close completely during fetal development. Both environmental and genetic factors have been seen to contribute to the cause of encephaloceles. Other causes include arsenic, tryptan blue, and teratogens.
Genes involved: Encephaloceles are more common in individuals who have a family history of neural tube defects such as spina bifida or anencephaly.
Pathogenesis: The sequence of events that lead to encephalocele is characterized by partial lacking of bone fusion. Hence, there is a gap through which a portion of the brain sticks out. Sometimes, the membranes that cover the brain along with the cerebrospinal fluid also protrudes out. The majority of encephaloceles are congenital.
Histology: The histology associated with encephalocele shows dysplastic nerve tissue of cerebral and cerebellar cortex.
How does Encephalocele Present?
Patients with encephalocele typically present at age range of 1 day to 6 years old. Males and females are equally affected. The symptoms, features, and clinical findings associated with encephalocele include seizures, loss of balance, ataxia, and loss of strength.
How is Encephalocele Diagnosed?
Encephalocele is diagnosed via prenatal ultrasound or physical exam. Other tests include fetal MRI, cell-free foetal DNA testing and amniocentesis.
How is Encephalocele Treated?
Encephalocele is treated with surgical intervention to place the protruding part of the brain and the membranes covering it back into the skull.
What is the Prognosis of Encephalocele?
The prognosis of encephalocele is poor if the patient develops complications.
What are Forebrain Anomalies?
Forebrain anomalies are malformations of the forebrain most commonly due to chromosomal abnormalities, fetal alcohol syndrome, and HIV-1 infection acquired in vitro.
Examples of forebrain anomalies include:
- Agenesis of the corpus callosum
- Holoprosencephaly
- Arrhinencephaly
- Microencephaly
- Macroencephaly
- Lissencephaly
- Polymicrogyria
- Neuronal heterotopias
What is Agenesis of the Corpus Callosum?
Agenesis of the corpus callosum is a rare birth defect in which there is a partial or complete absence of the corpus callosum. Agenesis of the corpus callosum results when the development of the corpus callosum is disrupted.
What is Holoprosencephaly?
Holoprosencephaly is a rare congenital brain malformation resulting from incomplete separation of the two hemispheres. The three main subtypes of holoprosencephaly in order of decreasing severity are:
- Alobar holoprosencephaly
- Semilobar holoprosencephaly
- Lobar holoprosencephaly
What is Arhinencephaly?
Arhinencephaly is a rare non-syndromic central nervous system malformation defined by the agenesis of the olfactory bulbs and tracts. Arhinecephaly causes congenital anosmia (lack of smell).
What is Microcephaly?
Microcephaly is a rare neurological condition in which an infant’s head is significantly smaller as compared with that of the other children of the same age and sex. Microcephaly can result from the brain developing abnormally in the womb or not growing as it should after birth. A variety of genetic and environmental factors can cause this microcephaly.
What is Macrocephaly?
Macrocephaly is a condition in which the head circumference is more than two standard deviations above the mean for gestational age and sex.
What is Lissencephaly?
Lissencephaly is a rare, gene-linked brain malformation characterized by the absence of normal folds and convolutions in the cerebral cortex and microcephaly. Children with lissencephaly usually have a normal sized head at birth.
What is Polymicrogyria?
Polymicrogyria is a condition characterized by abnormal development of the brain prior to birth. Polymicrogyria has too many unusually small folds.
What are Neuronal Heterotopias?
Neuronal heterotopias are brain malformations that result from deficits of neuronal migration. Individuals with neuronal heterotopias show a high incidence of neurological deficits, such as confusion, altered mental status, and epilepsy.
What are Posterior Fossa Anomalies?
Posterior fossa anomalies congenital abnormalities that represent a wide variety of disorders of malformations and disruptions.
Examples of posterior fossa anomalies include:
- Chiari type I malformation
- Chiari type II malformation
- Arnold-Chiari malformation
- Dandy-Walker malformation
- Joubert syndrome
What is Chiari Type I Malformation?
Chiari type I malformation is an abnormality in which the cerebellum bulges through a normal opening in the skull where it joins the spinal canal. This puts pressure on parts of the brain and spinal cord, and may cause mild to severe symptoms. In most cases, the problem is congenital. There are several types of Chiari malformations, but type I is the most common.
What is the Pathology of Chiari Type I Malformation?
Etiology: The cause of Chiari Type I malformation is unknown.
Genes involved: Genes that have been implicated in Chiari type I malformations include: HOX gene, Noggin gene, EFNB1, TBX6, FGF2, PAX
Pathogenesis: The sequence of events that lead to Chiari type 1 malformation are characterized by an inferior position of the cerebellar tonsils relative to foramen magnum. Some patients have an abnormal skull base or cervical segmentation abnormalities. Others have a small cranial vault or excess brain tissue.
Histology: The histology associated with Chiari type I malformation shows large and dysplastic fibrous tissue along with choroid plexus.
How does Chiari type I malformation Present?
Patients with Chiari type I malformation are more often females than males. The age range of the disease is mostly 25-45 years. The symptoms, features, and clinical findings associated with Chiari type 1 malformation include: sleep apnea, difficulty swallowing, muscle weakness, nystagus, headaches, and importantly the formation of a fluid filled pocket or cyst in the spinal cord, known as Syrinx.
How is Chiari type I malformation Diagnosed?
Chiari type I malformation is diagnosed mostly by MRI. A CT scan can also be done.
How is Chiari type I malformation Treated?
Chiari type I malformation can be treated with painkillers if symptoms are present. A surgery is done to relieve pressure on the brain and restore the flow of CSF.
What is the Prognosis of Chiari Type I Malformation?
The prognosis of Chiari type I malformation is generally fair after surgery and depends on the extent of neurological deficit.
What is Chiari Type II Malformation?
Chiari type II malformation is a relatively common congenital malformation of the spine and posterior fossa characterized by myelomeningocele and a small posterior fossa with the descent of the brainstem, vermis, and cerebellar tonsils.
What is the Pathology of Chiari Type II Malformation?
Etiology: The cause of Chiari type II malformation is unknown.
Pathogenesis: The sequence of events that lead to Chiari type II malformation is due to in utero malformation of the cranial structures and spine.
Histology: The histology associated with Chiari type II malformation shows dysplastic large fibrous tissue along with choroid plexus.
How does Chiari type II Malformation Present?
Patients with Chiari type II malformation are more often females than males, and the condition is congenital. The symptoms, features, and clinical findings associated with Chiari type II malformation include hydrocephalus, loss of strength, abnormal breathing, and depressed gag reflex.
How is Chiari type II Malformation Diagnosed?
Chiari type II malformation is diagnosed via MRI and CT scan.
How is Chiari type II Malformation Treated?
Chiari type II malformation is treated surgically.
What is the Prognosis of Chiari Type II Malformation?
The prognosis of Chiari type II malformation is poor.
What is Arnold-Chiari Malformation?
Arnold-Chiari malformation is a group of deformities of the posterior fossa and cerebellum, pons, and medulla oblongata.
What is the Pathology of Arnold-Chiari Malformation?
Etiology: The cause of Arnold Chiari malformation is primary or secondary. Primary cause includes primary congenital hypoplasia. Secondary causes include acquired morphologic changes, premature closure of sutures, calvarial dysplasia. Arnold-Chiari malformation can also be genetic.
Genes involved: Mutations on chromosomes 1 and 22
Pathogenesis: The sequence of events that lead to Arnold-Chiari malformation is abnormal brain development.
How does Arnold-Chiari Malformation Present?
Patients with Arnold Chiari malformation typically are females, and it most commonly it is congenital. The symptoms, features, and clinical findings associated with Arnold Chiari malformation include pain, muscle weakness, nystagmus, and fatigue.
How is Arnold Chiari Malformation Diagnosed?
Arnold Chiari malformation is diagnosed by radiographic imaging which may include ultrasound, CT scan, or MRI.
How is Arnold Chiari Malformation Treated?
Arnold Chiari malformation is treated with surgery.
What is the Prognosis of Arnold Chiari Malformation?
The prognosis of Arnold Chiari malformation is poor and mortality can be high in certain subtypes.
What is Dandy-Walker Malformation?
Dandy-Walker malformation is a rare congenital brain malformation in which the cerebellar vermis does not completely form.
What is the Pathology of Dandy-Walker Malformation?
Etiology: The cause of Dandy-Walker malformation is the disruption of embryonic development that affects the formation of the cerebellar vermis. This is usually a genetic mutation that results in impaired cell migration and division.
Genes involved: Trisomy 18 (Edward’s trisomy), FOXC1, NID1, ZIC1, ZIC4, FGF17, and LAMC1.
Pathogenesis: The sequence of events that lead to Dandy Walker malformation is the agenesis of the vermis, and cystic enlargement of the fourth ventricle which causes upward displacement of the tentorium and torcula.
How does Dandy Walker Malformation Present?
Patients with Dandy walker malformation are males and females of equal occurrence. The symptoms, features, and clinical findings associated with Dandy Walker syndrome include hydrocephalus, endocrine disorders, developmental delay, and eye abnormalities.
How is Dandy Walker Malformation Diagnosed?
Dandy walker malformation is diagnosed with the use of ultrasound, CT and MRI. Prenatal diagnosis of Dandy-Walker malformation is sometimes made by ultrasound or fetal MRI.
How is Dandy Walker Malformation Treated?
Dandy Walker malformation is treated according to the symptoms, whcih may include surgery and genetic counseling.
What is the Prognosis of Dandy Walker Malformation?
The prognosis of Dandy Walker malformation is poor and problems related to hydrocephalus are the most common cause of death.
What is Joubert Syndrome?
Joubert syndrome is a rare, autosomal recessive congenital cerebellar ataxia characterized by congenital malformation of the brainstem and agenesis or hypoplasia of the cerebellar vermis.
What is the Pathology of Joubert Syndrome?
Etiology: The cause of Joubert syndrome is an autosomal recessive genetic condition that causes problems with the structure and function of cilia.
Genes involved: OFD1, AHI1, NPHP1, and TMEM216
Pathogenesis: Genetic related improper formation of cilia.
How does Joubert Syndrome Present?
Patients with Joubert syndrome are typically males moreso than females. The symptoms, features, and clinical findings associated with Joubert syndrome include nystagmus, abnormal breathing, ataxia, and developmental delay.
How is Joubert Syndrome Diagnosed?
Joubert syndrome is diagnosed by an MRI scan, which shows the absence or underdevelopment of the cerebellar vermis.
How is Joubert syndrome Treated?
Joubert syndrome is treated symptomatically.
What is the Prognosis of Joubert syndrome?
The prognosis of Joubert syndromes is poor with an average lifespan of 8 years old. Respiratory failure is the most common cause of death.
What are Syringomyelia?
Syringomyelia is the development of a fluid-filled cyst (syrinx) within the spinal cord. Over time, the cyst can enlarge and destroy the spinal cord.
What is the Pathology of Syringomyelia?
Etiology: The causes of syringomyelia may include Chiari malformation, infections, and trauma.
Pathogenesis: The sequence of events that lead to Syringomyelia is the formation of a syrinx. When there is increased cerebrospinal fluid contained within the ependyma of the central canal of the spinal cord, it dissects the surrounding white matter to form this cystic cavity. A number of pathological conditions can cause an obstruction of the normal cerebrospinal fluid spaces, as discussed above.
Histology: The histology associated with syringomyelia shows cavitation of spinal cord gray matter and syrinx adjacent to the central canal.
How does Syringomyelia Present?
Patients with syringomyelia typically present between 20 and 40 years old, and men are more affected than women. The symptoms, features, and clinical findings associated with syringomyelia include muscle weakness, headaches, and stiffness.
How is Syringomyelia Diagnosed?
Syringomyelia is diagnosed via MRI.
How is Syringomyelia Treated?
Syringomyelia is treated with surgery.
What is the Prognosis of Syringomyelia?
The prognosis of syringomyelia depends on the underlying cause.
What is Hydromyelia?
Hydromyelia is dilatation of the central canal of the spinal cord.
What is the Pathology of Hydromyelia?
Etiology: The causes of hydromyelia includes infections, spinal tumors, and trauma.
Pathogenesis: The sequence of events that lead to hydromyelia is due to the abnormal widening of the central canal of the spinal cord that allows the buildup of fluid.
How does Hydromyelia Present?
Patients with hydromyelia typically are males rather than females with an age range of 25-40 years. The symptoms, features, and clinical findings associated with hydromyelia include loss of feeling in the hands and arms, leg pain, and muscle weakness.
How is Hydromyelia Diagnosed?
Hydromyelia is diagnosed with MRI. Additional tests include electromyography, CT scan, and myelogram.
How is Hydromyelia Treated?
Hydromyelia is treated by surgery and physical therapy.
What is the Prognosis of Hydromyelia?
The prognosis of hydromyelia is good and it may rarely resolve on its own without medical intervention. Surgery may permanently or temporarily relieve symptoms, but it can also cause a number of severe complications.
What are Perinatal Brain Injuries?
Perinatal brain injuries are injuries to the vulnerable, developing fetal brain such as cerebral palsy.
What is Cerebral Palsy?
Cerebral palsy is a group of disorders that affect a person’s ability to move and maintain balance and posture. Cerebral palsy is the most common motor disability in childhood.
What is the Pathology of Cerebral Palsy?
Etiology: The causes of cerebral palsy comprise all those conditions which may lead to abnormal development of the brain or damage to it which may occur during pregnancy, delivery, the first month of life, or less commonly in early childhood.
Genes involved: Unknown.
Pathogenesis: The sequence of events that lead to cerebral palsy include the abnormal development of the brain or damage to the developing brain that affects a child’s ability to control his or her muscles.
How does Cerebral Palsy Present?
Patients with cerebral palsy are mostly infants or children in preschool years. It is more common in boys than girls. The symptoms, features, and clinical findings associated with cerebral palsy include abnormal movements and variations in muscle tones.
How is Cerebral Palsy Diagnosed?
Cerebral palsy is diagnosed by physical exam.
How is Cerebral Palsy Treated?
Cerebral palsy may be treated by botulinum toxin injections and other muscle relaxants such as baclofen, tizanidine (Zanaflex), diazepam (Valium) or dantrolene (Dantrium). Physical therapy may be recommended, as well as surgical procedures to correct any bone abnormalities.
What is the Prognosis of Cerebral Palsy?
The prognosis of cerebral palsy is good if it is mild, and the child can live up to 20 years. However, as the severity increases, the prognosis becomes poor.
What is Trauma of the Central Nervous System?
Trauma of the central nervous system is injury to the brain or spinal cord. This is known as traumatic brain injury (TBI) and spinal cord injury (SCI). Trauma pushes the activation of the nervous system beyond its ability to self-regulate.
What is Cerebrovascular Disease?
Cerebrovascular disease refers to a group of conditions that affect blood flow and the blood vessels in the brain. Problems with blood flow may occur from blood vessels narrowing (stenosis), clot formation (thrombosis), artery blockage (embolism), or blood vessel rupture (hemorrhage).
Examples of cerebrovascular disease include:
- Hypoxia
- Ischemia: global, focal, or secondary to infarction
- Hypertensive cerebrovascular disease: such as hypertensive encephalopathy
What are Prion Diseases?
Prion diseases are or transmissible spongiform encephalopathies that can cause rare progressive neurodegenerative disorders that affect both humans and animals. Prion diseases are usually rapidly progressive and fatal.
Examples of Prion Diseases include:
- Creutzfeldt-Jakob Diseases
- Kuru
- Gerstman-Straussler-Scheinker Syndrome
- Fatal Familial Insomnia
What is Creutzfeldt-Jakob disease?
Creutzfeldt-Jakob disease is a transmissible spongiform encephalopathy that results in rapidly progressive dementia and death usually within a year from onset. The vast majority are sporadic, but familial and acquired forms are occasionally encountered.
What is the Pathology of Creutzfeldt-Jakob disease?
Etiology: The cause of Creutzfeldt-Jakob disease are prions. They are misfolded proteins that occur in the neurons of the central nervous system (CNS). They are thought to affect signalling processes, damaging neurons and resulting in degeneration that causes the spongiform appearance in the affected brain.
Genes involved: People can also develop CJD because they carry a mutation of the gene that codes for the prion protein (PRNP).
Pathogenesis: There are four types of Creutzfeldt-Jakob disease:
- Sporadic (sCJD)
- Variant (vCJD)
- Familial (fCJD)
- Iatrogenic (iCJD)
Histology: The histology associated with Creutzfeldt-Jakob disease shows sponge-like lesions in the brain tissue. There is also the presence of many round vacuoles from one to 50 micrometres in the neuropil, which appear glassy or eosinophilic and may coalesce. Neuronal loss and gliosis is may also be seen.
How does Creutzfeldt-Jakob Disease Present?
Patients with Creutzfeldt-Jakob disease typically affects more males than females. It is frequently found in people 55-65 years of age. The symptoms, features, and clinical findings associated with Creutzfeldt-Jakob disease include dementia, hallucinations, anxiety, depression, and ataxia.
How is Creutzfeldt-Jakob Disease Diagnosed?
Creutzfeldt-Jakob disease is diagnosed by history, physical exam, electroencephalography, MRI, CSF fluid analysis, and brain biopsy.
How is Creutzfeldt-Jakob Disease Treated?
Creutzfeldt-Jakob disease has no cure. Treatment is mostly palliative. Sedatives and antidepressants may help with symptoms. Myoclonic jerks can be handled with clonazepam or sodium valproate.
What is the Prognosis of Creutzfeldt-Jakob Disease?
The prognosis of Creutzfeldt-Jakob disease is poor.
What is Kuru?
Kuru is a rare, incurable and fatal neurodegenerative disorder caused by the transmission of abnormally folded proteins called prions.
What is the Pathology of Kuru?
Etiology: Kuru is an acquired infectious disease linked cannibalism.
Pathogenesis: A chain reaction of misfolded proteins that affect the brain.
Histology: The histology associated with Kuru shows neuronal loss, spongiform change, and astrogliosis. Amyloid plaques known as “kuru plaques” are also seen.
How does Kuru Present?
Patients with Kuru typically have an age range of 20-45 years. Canabillistic men and women are equally affected. The symptoms, features, and clinical findings associated with Kuru include body tremors, cerebellar ataxia, dysarthria, dysphagia, and pathologic bursts of laughter.
How is Kuru Diagnosed?
Kuru is diagnosed mainly through history and physical examination. The characteristic progression of symptoms in the vulnerable populations leads to high suspicion of the diagnosis. There are no laboratory and imaging tests available that give a definitive diagnosis of kuru. The distinct histopathological findings are diagnostic.
How is Kuru Treated?
There is no known treatment for kuru.
What is the Prognosis of Kuru?
The prognosis of Kuru is poor and death usually occurs within 1 year after the first sign of symptoms.
What is Gerstman-Straussler-Scheinker Syndrome?
Gerstman-Straussler-Scheinker syndrome is an extremely rare, usually familial inherited fatal neurodegenerative disease.
What is the Pathology of Gerstman-Straussler-Scheinker Syndrome?
Etiology: The cause of Gerstman-Straussler-Scheinker Syndrome are the prions, which are a class of pathogenic proteins that are resistant to proteases. These prions then form clusters in the brain, which are responsible for the neurodegenerative effects seen in patients.
Genes involved: Point mutations in the PRNP gene.
Pathogenesis: The prion proteins (PrP) are produced by the prion protein gene (PRNP gene) do not get degraded, and cause more misfolded proteins that gives rise to the disease.
How does Gerstman-Straussler-Scheinker Syndrome Present?
Patients with Gerstman-Straussler-Scheinker Syndrome have an average age range of 35-50 years. Males and females are equally affected. The symptoms, features, and clinical findings associated with Gerstman-Straussler-Scheinker Syndrome include dementia, dysarthria, nystagmus, truncal ataxia, and parathesia.
How is Gerstman-Straussler-Scheinker Syndrome Diagnosed?
Gerstman-Straussler-Scheinker syndrome is diagnosed by genetic testing.
How is Gerstman-Straussler-Scheinker Syndrome Treated?
Gerstman-Straussler-Scheinker syndrome has no cure.
What is the Prognosis of Gerstman-Straussler-Scheinker Syndrome?
The prognosis of Gerstman-Straussler-Scheinker Syndrome is poor.
What is Fatal Familial Insomnia?
Fatal familial insomnia is a rare genetic prion disorder that results in trouble sleeping.
What is the Pathology of Fatal Familial Insomnia?
Etiology: The cause of Fatal Familial Insomnia is the gene PRNP that provides instructions for making the prion protein.
Genes involved: PRNP gene.
Pathogenesis: Reduced glucose use by the thalamus and a mild hypo-metabolism of the cingulate cortex due to the prion proteins.
How does Fatal Familial Insomnia Present?
Patients with Fatal Familial Insomnia have an average age of onset of 50 years with an age range of 18-60 years. It is equally common among men and women. The symptoms, features, and clinical findings associated with fatal familial insomnia include insomnia, hallucinations, dementia, and neck stiffness.
How is Fatal Familial Insomnia Diagnosed?
Fatal Familial Insomnia is diagnosed on the basis of symptoms and genetic testing.
How is Fatal Familial Insomnia Treated?
Fatal familial insomnia is treated with symptomatic care.
What is the Prognosis of Fatal Familial Insomnia?
The prognosis of Fatal Familial Insomnia is poor.
What are Demyelinating Diseases?
Demyelinating diseases are conditions that result in damage to the myelin sheath that surrounds nerve fibers. Damage to the myelin sheath can result in delayed or even absent transmission of nerve impulses which may present as neurological problems.
Examples of demyelinating diseases include:
- Acquired degeneration of myelin
- Multiple sclerosis
- Neuromyelitis optica
- Acute disseminated encephalomyelitis
- Acute necrotizing hemorrhagic encephalomyelitis
- Central pontine myelinolysis
What is Acquired Degeneration of Myelin?
Acquired degeneration of myelin is the one that begins at some point during one’s lifetime, as opposed to disease that was already present at birth, which is known as congenital disease.
What is Multiple Sclerosis?
Multiple sclerosis is autoimmune inflammatory disease that attacks myelinated axons.
What is the Pathology of Multiple Sclerosis?
Etiology: The cause of Multiple Sclerosis is unknown.
Genes involved: HLA region of Chromosome 6. Changes in this area increase the probability of getting MS.
Pathogenesis: The sequence of events that lead to Multiple Sclerosis are the formation of lesions in the central nervous system (also called plaques) on myelin due to autoimmune attack.
Histology: The histology associated with Multiple Sclerosis shows multiple focal areas of myelin loss within the CNS called plaques or lesions, macrophages, and gliosis.
How does Multiple Sclerosis Present?
Patients with Multiple Sclerosis are most common in the age range 20-40 years. Females are more commonly affected. The symptoms, features, and clinical findings associated with multiple sclerosis include dizziness, fatigue, movement issues, and eye issues.
How is Multiple Sclerosis Diagnosed?
Multiple Sclerosis is diagnosed primarily by ruling out other conditions and looking at the history and clinical findings. Tests include evoked potential, laboratory tests, spinal tap, and MRI.
How is Multiple Sclerosis Treated?
Multiple sclerosis has no cure, and is symptomatically managed.
What is the Prognosis of Multiple Sclerosis?
The prognosis of Multiple Sclerosis is good. Most people with MS have a relapsing-remitting disease course
What is Neuromyelitis Optica?
Neuromyelitis optica is bilateral optic neuritis in the setting of demyelination of the spinal cord. Neuromyelitis optica is also known as Devic’s disease. Neuromyelitis optica occurs when your the body’s immune system reacts against its own cells in the central nervous system, mainly in the optic nerves and spinal cord, but sometimes in the brain as well.
What is Acute Disseminated Encephalomyelitis?
Acute disseminated encephalomyelitis is is a neurological, immune-mediated disorder in which widespread inflammation of the brain and spinal cord. This damages the white matter.
What is Acute Necrotizing Hemorrhagic Encephalomyelitis?
Acute necrotizing hemorrhagic encephalomyelitis is a rare, demyelinating disease of the CNS characterized by rapidly progressive inflammation of the white matter. It is the most severe form of acute disseminated encephalomyelitis.
What is Central Pontine Myelinolysis?
Central pontine myelinolysis is a neurological disorder that most frequently occurs after too rapid medical correction of sodium deficiency. The rapid rise in sodium concentration is accompanied by the movement of small molecules and pulls water from brain cells. The shift in water and brain molecules leads to the destruction of myelin. Certain areas of the brain are particularly susceptible to myelinolysis, especially the pons.
What are Neurodegenerative Diseases?
Neurodegenerative diseases are degenerative nerve diseases that affect balance, movement, talking, breathing, and heart function. Many of these diseases are genetic. Most of them have no cure.
Examples of neurodegenerative diseases include:
- Alzheimer disease
- Frontotemporal degeneration
- Parkinson disease
- Atypical parkinsonism syndromes
- Multiple system atrophy
- Huntington disease
- Spinocerebellar degenerations
- Amyotrophic lateral sclerosis
- Other motor neuron diseases
What is Alzheimer Disease?
Alzheimer disease is a neurodegenerative disorder marked by cognitive and behavioral impairment that significantly interferes with social and occupational functioning. It is an incurable disease with a long preclinical period and progressive course.
What is the Pathology of Alzheimer Disease?
Etiology: The etiology of Alzheimer disease is unknown.
Genes involved: Amyloid precursor protein (APP) at chromosome 21, presenilin (PS) 1 and 2 (chromosome 14 and 1), and the gene encoding apolipoprotein E (ApoE) linked to chromosome 19 is a risk factor for late-onset AD.
Pathogenesis: The sequence of events that lead to Alzheimer disease is the alteration in one or more aspects of beta amyloid, leading to proliferation of extracellular amyloid plaques. Another pathway involves alteration in one or more aspects of tau protein metabolism, leading to intracellular neurofibrillary tangles. As part of these processes, an inflammatory cascade causes oxidative neuronal injuries and depletion of several neurotransmitters.
Histology: The histology associated with Alzheimer disease shows extracellular amorphus eosinophilic deposits of amyloid consisting of Aβ peptides, which are referred to as amyloid plaques and neurofibrillary tangles which are intraneuronal aggregates of abnormally modified microtubule-associated protein tau.
How does Alzheimer Disease Present?
Patients with Alzheimer disease typically are older. It has a higher occurrence in women. The symptoms, features, and clinical findings associated with Alzheimer disease include memory loss, poor judgment, and mood changes.
How is Alzheimer Disease Diagnosed?
Alzheimer disease is diagnosed by clinical and physical exam.
How is Alzheimer Disease Treated?
Alzheimer disease has no cure. Some medications may help with symptoms such as Acetylcholinesterase inhibitors (donepezil, galantamine and rivastigmine). Other treatments include cognative rehabilitation and cognitive stimulation.
What is the Prognosis of Alzheimer disease?
The prognosis of Alzheimer disease fair.
What is Frontotemporal Degeneration?
Frontotemporal degeneration is a group of disorders that occur when nerve cells in the frontal and temporal lobes of the brain are lost.
What is the Pathology of Frontotemporal Degeneration?
Etiology: The cause of Frontotemporal degeneration is unknown.
Genes involved: Mutations in the MAPT gene on chromosome 17.
Pathogenesis: Frontal lobe neuronal degeneration.
Histology: The histology associated with Frontotemporal degeneration shows four major types of features microvacuolation without neuronal inclusions, and gliosis.
How does Frontotemporal Degeneration Present?
Patients with frontotemporal degeneration typically are typically older. It is equally common in males and females. The signs and symptoms include abnormal social behavior, lack of inhibition, lack of judgement, and tremors.
How is Frontotemporal Degeneration Diagnosed?
Frontotemporal degeneration is diagnosed by hitsory and physical exam.
How is Frontotemporal Degeneration Treated?
Frontotemporal degeneration has no cure. Antidepressants and antipsychotics may be helpful.
What is the Prognosis of Frontotemporal Degeneration?
The prognosis of Frontotemporal degeneration is poor.
What is Parkinson Disease?
Parkinson disease is a neurodegenerative disorder that affects predominately dopaminergic neurons in the substantia nigra.
What is the Pathology of Parkinson Disease?
Etiology: The cause of Parkinson disease the loss of nerve cells in the substantia nigra.
Genes involved: Parkin, PINK1, Mutations in α-synuclein, UCHL1, and DJ1
Pathogenesis: The pathology of Parkinson’s disease is loss of dopamine cells in the substantia nigra.
Histology: The histology associated with Parkinson disease shows Lewy Body, a cytoplasmic aggregate of proteins that appears eosinophilic, round, and elongated. There is also the presence of macrophages with pigmented material. These represent sites of neurodegeneration.
How does Parkinson Disease Present?
Patients with Parkinson disease typically are over 60 years of age. It is more common in men than women. The symptoms, features, and clinical findings associated with Parkinson disease include tremors, rigidity, bradykinesia, and impaired gait.
How is Parkinson Disease Diagnosed?
Parkinson disease is diagnosed based on the medical history, signs and symptoms, and a neurological and physical examination.
How is Parkinson Disease Treated?
Parkinson disease has no cure. Medications that may help include Carbidopa-levodopa, and dopamine agonists like pramipexole, or ropinirole. Amantadine may be helpful as well.
What is the Prognosis of Parkinson Disease?
The prognosis of Parkinson disease is fair. Most people with Parkinson’s disease now have a normal or near-normal life expectancy.
What are Atypical Parkinsonism Syndromes?
Atypical parkinsonism syndromes are progressive diseases that present with some of the signs and symptoms of Parkinson’s disease, but do not respond well to drug treatment with levodopa. Aytpical parkinsonism syndromes are associated with abnormal protein buildup within brain cells.
Examples of atypical parkinsonism syndromes include:
- Progressive supranuclear palsy
- Corticobasal degeneration
What is Progressive Supranuclear Palsy?
Progressive supranuclear palsy is a rare brain disorder that causes problems with movement, walking and balance, and eye movement. Progressive supranuclear palsy results from damage to nerve cells in the brain that control thinking and body movement.
What is the Pathology of Progressive Supranuclear Palsy?
Etiology: The cause of progressive supranuclear palsy is a variant in the gene for tau protein called the H1 haplotype, located on chromosome 17.
Genes involved: the gene for tau protein called the H1 haplotype, located on chromosome 17.
Pathogenesis: Neuronal loss.
Histology: The histology associated with progressive supranuclear palsy shows neuronal loss, neurofibrillary tangles and gliosis.
How does Progressive Supranuclear Palsy Present?
Patients with progressive supranuclear palsy are typically of 60–70 years of age. Males are slightly more likely to be affected than females. The symptoms, features, and clinical findings associated with Progressive supranuclear palsy include vision disturbances, balance problems, and movement issues.
How is Progressive Supranuclear Palsy Diagnosed?
Progressive supranuclear palsy is diagnosed by ruling out other conditions.
How is Progressive Supranuclear Palsy Treated?
Progressive supranuclear palsy has no cure.
What is the Prognosis of Progressive Supranuclear Palsy?
The prognosis of progressive supranulcear palsy is poor.
What is Corticobasal Degeneration?
Corticobasal degeneration is a progressive neurological disorder characterized by nerve cell loss and atrophy of multiple areas of the brain including the cerebral cortex and the basal ganglia.
What is the Pathology of Corticobasal Degeneration?
Etiology: The exact, underlying cause of corticobasal degeneration is unknown.
Pathogenesis: Progressive deterioration of tissue in different parts of the brain.
Histology: The histology associated with corticobasal degeneration shows presence of neuronal cytoplasmic inclusions.
How does Corticobasal Degeneration Present?
Patients with corticobasal degeneration typically occurs in age range 50-70 years and affects more females than males. The symptoms, features, and clinical findings associated with Corticobasal degeneration include progressive asymmetric rigidity, pain, apraxia, bradykinesia, dystonia, and myoclonus.
How is Corticobasal Degeneration Diagnosed?
Corticobasal degeneration is diagnosed based on signs and symptoms.
How is Corticobasal Degeneration Treated?
There are no treatments that help slow the progression of corticobasal degeneration.
What is the Prognosis of Corticobasal Degeneration?
The prognosis of Corticobasal degeneration is poor, and the disease progresses over time.
What is Multiple System Atrophy?
Multiple system atrophy is a progressive neurodegenerative disorder with a combination of symptoms that affect both the autonomic nervous system and movement.
What is the Pathology of Multiple System Atrophy?
Etiology: The cause of Multiple System Atrophy is unknown. Multiple environmental and genetic factors are important.
Genes involved: Deletion of SHC2 gene.
Pathogenesis: The sequence of events that lead to multiple system atrophy is characterized by progressive loss of neurons in various structures of the brain.
Histology: Cell loss and gliosis or a proliferation of astrocytes in damaged areas of the central nervous system. A scar may also be present.
How does Multiple System Atrophy Present?
Patients with multiple system atrophy typically are predominantly males, with the most common age group of 50-60 years. The symptoms, features, and clinical findings associated with Multiple System Atrophy include ataxia, constipation, sleep apnea, and cognitive impairment.
How is Multiple System Atrophy Diagnosed?
Multiple system atrophy is diagnosed by MRI and CT scan. Pathological diagnosis can only be made at autopsy by finding abundant GCIs on histologic analysis.
How is Multiple System Atrophy Treated?
Multiple system atrophy is treated by symptom management and avoidance of postural hypotension.
What is the Prognosis of Multiple System Atrophy?
The prognosis of multiple system atrophy is poor.
What is Huntington Disease?
Huntington disease is a rare, inherited disease that causes the progressive breakdown of nervous cells in the brain. This disease causes changes in the central area of the brain, which affect movement, mood and thinking skills.
What is the Pathology of Huntington Disease?
Etiology: The cause of Huntington disease is a mutation in the gene for a protein called huntingtin.
Genes involved: A single defective gene on chromosome 4. This defect is dominant so whoever inherits it from a parent with Huntington’s will eventually develop the disease.
Pathogenesis: The sequence of events that lead to Huntington disease is repetitive expansion of CAG that results in aberrant huntingtin protein.
Histology: The histology associated with Huntington disease shows neuronal loss, atrophy, and gliosis of the caudate and putamen beginning in the anterior medial caudate.
How does Huntington Disease Present?
Patients with Huntington disease are typically between 30-50 years. Males and females have the same risk of inheriting the disease. The symptoms, features, and clinical findings associated with Huntington disease include depression, difficulty concentrating, depression, personality changes, and involuntary jerking.
How is Huntington Disease Diagnosed?
Huntington disease is diagnosed by history and physical exam.
How is Huntington Disease Treated?
Huntington disease is not curable. Medications may lessen some symptoms of movement and psychiatric disorders.
What is the Prognosis of Huntington Disease?
The prognosis of Huntington disease is poor and disability gets worse over time.
What are Spinocerebellar Degenerations?
Spinocerebellar degenerations are a group of hereditary ataxias that are characterized by degenerative changes in the cerebellum, and sometimes in the spinal cord.
What is the Pathology of Spinocerebellar Degenerations?
Etiology: The cause of Spinocerebellar degenerations may be genetics.
Genes involved: Mutations in many different genes are known to cause the different types of Spinocerebellar degenerations. Some types that are inherited in an autosomal dominant manner are caused by trinucleotide repeat expansions.
Pathogenesis: Several types of spinocerebellar degenerations are characterized by repeat expansion of the trinucleotide sequence CAG.
How does Spinocerebellar Degenerations Present?
Patients with Spinocerebellar degenerations are from 25-80 years of age typically, and have equal male and female predominance. The symptoms, features, and clinical findings associated with Spinocerebellar degenerations include ataxia, abnormal gait, poor coordination, and vision problems.
How is Spinocerebellar Degenerations Diagnosed?
Spinocerebellar degenerations may be diagnosed by history, physical exam, and genetic tests.
How is Spinocerebellar Degenerations Treated?
There is no known cure for Spinocerebellar degenerations.
What is the Prognosis of Spinocerebellar Degenerations?
The prognosis of Spinocerebellar degenerations is poor.
What is Amyotrophic Lateral Sclerosis?
Amyotrophic lateral sclerosis is a progressive nervous system disease that affects nerve cells in the brain and spinal cord, causing loss of muscle control. ALS is also known as Lou Gehrig’s disease, after the baseball player who was diagnosed with it.
What is the Pathology of Amyotrophic Lateral Sclerosis?
Etiology: The cause of amyotrophic lateral sclerosis is genetic in 5% to 10% of the people. For the rest, the cause isn’t known.
Genes involved: Unknwn.
Pathogenesis: The sequence of events that lead to Amyotrophic lateral sclerosis is upper motor neuron and lower motor neuron signs.
Histology: The histology associated with amyotrophic lateral sclerosis shows ubiquinated inclusions in lower motor neurons and axonal swellings that are thought to contain disarrayed neurofilaments.
How does Amyotrophic Lateral Sclerosis Present?
Patients with amyotrophic lateral sclerosis are usually males before the age of 65 years old. After 70 years, there is no sex difference. The symptoms, features, and clinical findings associated with Amyotrophic lateral sclerosis include difficulty walking, weakness, slurred speech, cramps, and clumsiness.
How is Amyotrophic Lateral Sclerosis Diagnosed?
Amyotrophic lateral sclerosis is diagnosed by history, physical examination, and electromyography (EMG).
How is Amyotrophic Lateral Sclerosis Treated?
Amyotrophic lateral sclerosis is symptomatically managed. The medication Riluzole may be helpful.
What is the Prognosis of Amyotrophic Lateral Sclerosis?
The prognosis of amyotrophic lateral sclerosis is poor and the disease is fatal.
What are Other Motor Neuron Diseases?
Other motor neuron diseases not mentioned above include:
- Primary lateral sclerosis
- Progressive bulbar palsy
- Pseudobulbar palsy
- Progressive muscular atrophy
- Spinal muscular atrophy
- Kennedy’s disease
What are Metabolic Diseases of the Central Nervous System?
Metabolic diseases of the central nervous system are genetically associated abnormalities of enzymes. The metabolic consequences of defective enzymes affect the development or functioning of the nervous system. Although most metabolic diseases of the central nervous system occur in children, some can present in adult life.
Examples of metabolic diseases of the central nervous system include:
- Leukodystrophies
- Neuronal storage disease
- Mitochondrial encephalomyopathies
What are Leukodystrophies?
Leukodystrophies are diseases affect myelin in the brain and spine.
Examples of Leukodystrophies include:
- Adrenoleukodystrophy
- Krabbe disease
- Metachromatic leukodystrophy
What is Adrenoleukodystrophy?
Adrenoleukodystrophy is a type of genetic condition that damages the myelin sheath. Forms of X-linked adrenoleukodystrophy include:
- Childhood-onset adrenoleukodystrophy
- Addison’s disease
- Adrenomyeloneuropathy
What is the Pathology of Adrenoleukodystrophy?
Etiology: The cause of adrenoleukodystrophy genetic. It is an X-linked recessive condition caused by a mutation in the ABCD1 gene on the X chromosome. Because a female has two X chromosomes, if she inherits the faulty gene, then she still has another X chromosome to offset the mutation.
Genes involved: Mutations in the ABCD1 gene.
Pathogenesis: The sequence of events that lead to adrenoleukodystrophy is the mutations in the ABCD1 gene, which causes accumulation of very long-chain fatty acids in plasma and tissues.
How does Adrenoleukodystrophy Present?
Patients with Adrenoleukodystrophy are predominantly boys between ages 4 and 10. The symptoms, features, and clinical findings associated with Adrenoleukodystrophy include
Learning issues, vision issues, and coordinationissues. Virtually all males with adrenoleukodystrophy develop adrenal insufficiency and myelopathy.
How is Adrenoleukodystrophy Diagnosed?
Adrenoleukodystrophy is diagnosed by by physical exam and laboratory tests to check for high levels of very long-chain fatty acids in the blood.
How is Adrenoleukodystrophy Treated?
Adrenoleukodystrophy has no cure. Stem cell transplantation may be considered. Corticosteroids and physical therapy may be helpful.
What is the Prognosis of Adrenoleukodystrophy?
The prognosis of Adrenoleukodystrophy is generally poor due to progressive neurological deterioration unless bone marrow transplantation is done.
What is Krabbe Disease?
Krabbe disease is an inherited disorder that destroys myelin in the brain and throughout the nervous system. Krabbe disease is due to deficiency of the enzyme galactocerebrosidase which is needed for the breakdown of the sphingolipids.
What is the Pathology of Krabbe Disease?
Etiology: The cause of Krabbe disease is the failure to break down the sphingolipids, resulting in degeneration of the myelin sheath.
Genes involved: Unknown.
Pathogenesis: In the case of Krabbe disease, two mutated copies of a particular gene result in little or no production of an enzyme called galactocerebrosidase.
Histology: The histology associated with Krabbe disease shows pathognomonic PAS+ globoid macrophages, extensive myelin and oligodendrocyte loss.
How does Krabbe Disease Present?
Patients with Krabbe disease typically are infants during the first 2 to 5 months of life. Males and females are equally affected. Common signs and symptoms early in the course of the disease include the following feeding difficulties, irritability, seizures, and frequent vomiting.
How is Krabbe Disease Diagnosed?
Krabbe disease is diagnosed by blood testing to assess the level of GALC enzyme activity.
How is Krabbe Disease Treated?
Krabbe disease has no cure if the infant is diagnosed with it. Treatment options focus on managing symptoms and providing supportive care.
What is the Prognosis of Krabbe Disease?
The prognosis of Krabbe disease is poor. On average, infants who develop Krabbe disease will die before 2 years old.
What is Metachromatic Leukodystrophy?
Metachromatic leukodystrophy is an inherited lysosomal storage diseases. Metachromatic leukodystrophy involves a progressive deterioration of motor and neurocognitive function.
What is the Pathology of Metachromatic Leukodystrophy?
Etiology: The cause of metachromatic leukodystrophy is deficient activity of arylsulfatase A, along with genetic disturbances.
Genes involved: Arylsulfatase A gene (ARSA gene), on chromosome 22q13.3.
Pathogenesis: Inability to degrade sulfated glycolipids, mainly the galactosyl-3-sulfate ceramides.
Histology: The histology associated with metachromatic leukodystrophy shows metachromatic granules. In the nervous system, the loss of myelinated oligodendrocytes is seen.
How does Metachromatic Leukodystrophy Present?
Patients with metachromatic leukodystrophy are on average 4 years old. Some cases are in the age range 12-14 years. The disease has equal occurrence in both sexes. The symptoms, features, and clinical findings associated with metachromatic leukodystrophy include loss of the ability to detect sensations, hearing loss, seizures, and psychosis.
How is Metachromatic Leukodystrophy Diagnosed?
Metachromatic leukodystrophy is diagnosed by laboratory testing.
How is Metachromatic Leukodystrophy Treated?
Metachromatic leukodystrophy currently has no treatment. Symptomatic supportive care is needed to address constipation, seizures, dystonias, spasticity, and difficulty feeding.
What is the Prognosis of Metachromatic Leukodystrophy?
The prognosis of metachromatic leukodystrophy is poor. Most children within the infantile form die before 6 years old.
What are Neuronal Storage Diseases?
Neuronal storage diseases are storage diseases in the central nervous system that result from a deficiency of a specific degradative lysosomal enzyme causing the accumulation of a substrate that is stored in the cytoplasm of the neuronal cell body, and occasionally in glia, macrophages, and the cells of other organs.
Examples of neuronal storage diseases include:
- Niemann-Pick disease
- Tay-Sachs disease
What is Niemann-Pick Disease?
Niemann-Pick disease is a group of severe inherited metabolic disorders, in which sphingomyelin accumulates in lysosomes in cells.
What is the Pathology of Niemann-Pick Disease?
The pathology of Niemann-Pick disease is:
Etiology: The cause of Niemann-Pick disease is genetic. It is inherited in an autosomal recessive pattern.
Genes involved: Mutations in the SMPD1 gene, NPC1, or NPC2.
Pathogenesis: Deficiency of sphingomyelinase that results in lack of myelin.
Histology: The histology associated with Niemann-Pick disease shows lipid-laden macrophages in the marrow and sea-blue histiocytes on. Numerous small vacuoles of relatively uniform size are causing a foamy appearance may be seen.
How does Niemann-Pick Disease Present?
Patients with Niemann-Pick disease typically are children. It is present in males and females equally. The symptoms, features, and clinical findings associated with Niemann-Pick disease include hepatomegaly, ataxia, dystonia, dysarthria, and sleep issues.
How is Niemann-Pick Disease Diagnosed?
Niemann-Pick disease is diagnosed by by laboratory testing.
How is Niemann-Pick Disease Treated?
Niemann-Pick disease is treated with cholesterol medications like statins, and potentially bone marrow transplants.
What is the Prognosis of Niemann-Pick Disease?
The prognosis of Niemann-Pick disease is fair. Niemann Pick disease Type A is typically fatal before 3 years of age. In Type B, mortality before adulthood is common as well. But many patients live well into adulthood and may reach a normal lifespan. Type C has a variable prognosis.
What is Tay-Sachs Disease?
Tay-Sachs disease is a rare, neurodegenerative disorder in which deficiency of the enzyme hexosaminidase A which results in excessive accumulation of gangliosides in the brain and nerve cells.
What is the Pathology of Tay-Sachs Disease?
Etiology: The cause of Tay-Sachs disease a mutation in the hexosaminidase subunit alpha (HEXA) gene.
Genes involved: Tay–Sachs disease results from mutations in the HEXA gene on chromosome 15.
Pathogenesis: Failure to breakdown GM2-ganglioside results in its abnormal accumulation in brain and nerve cells eventually resulting in the progressive deterioration of the central nervous system.
How does Tay-Sachs Disease Present?
Patients with Tay-Sachs disease typically are categorized according to the type of disease. The age range varies between 6 months to 40 years old, and common equally among males and females. The symptoms, features, and clinical findings associated with Tay-Sachs disease include paralysis, startle response, vision issues, and ataxia.
How is Tay-Sachs Disease Diagnosed?
Tay-Sachs disease is diagnosed by enzyme assay to measure the activity of hexosaminidase.
Molecular analysis may be useful. Ophthalmoscopy showing a cherry red macula is pathognomonic for Tay-Sachs disease..
How is Tay-Sachs Disease Treated?
Tay-Sachs disease is treated with supportive care.
What is the Prognosis of Tay-Sachs Disease?
The prognosis of Tay-Sachs disease is poor. Tay-Sachs disease is a progressive disease that gets worse over time.
What are Mitochondrial Encephalomyopathies?
Mitochondrial encephalomyopathies represent a clinically heterogeneous group of disorders resulting from abnormal mitochondrial function. Organs with high mitochondrial loads (like the brain and muscles) are particularly susceptible to mitochondrial dysfunction.
Examples of mitochondrial encephalomyopathies include:
- Leigh syndrome
- Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS)
- Myoclonic epilepsy and ragged red fibers (MERRF)
What is Leigh Syndrome?
Leigh syndrome is a rare genetic neurometabolic disorder that is characterized by the degeneration of the central nervous system.
What is the Pathology of Leigh syndrome?
Etiology: The cause of Leigh syndrome is mutation in mitochondrial DNA or by deficiencies of an enzyme called pyruvate dehydrogenase.
Genes involved: MT-ATP6, SURF1.
Pathogenesis: The sequence of events that lead to Leigh syndrome are oxidative phosphorylation deficiencies.
Histology: The histology associated with Leigh syndrome shows vacuolation of the neuropil in a background of relative neuronal preservation, associated with demyelination, gliosis, and vascular proliferation and thickening.
How does Leigh Syndrome Present?
Patients with Leigh syndrome are in the age range 3 months to 2 years and affects more males than females. The symptoms, features, and clinical findings associated with Leigh syndrome include nystagmus, developmental delay, and ataxia.
How is Leigh Syndrome Diagnosed?
Leigh syndrome is diagnosed by using the following criteria physical exam and genetic tests.
How is Leigh Syndrome Treated?
Leigh syndrome is treated specifically to minimize the symptoms. Supportive care for Leigh syndrome includes treatment of acidosis, seizures, dystonia, and cardiomyopathy, and attention to nutritional status. Evaluations with a neurologist, ophthalmologist, audiologist, and cardiologist are recommended.
What is the Prognosis of Leigh Syndrome?
The prognosis of Leigh syndrome is poor.
What is Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-like Episodes (MELAS)?
Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS) is an extremely rare genetic condition that begins in childhood. The disorder affects many areas of the body, especially the brain and nervous system. MELAS is also characterized by a buildup of lactic acid in the body as well as stroke-like symptoms, such as temporary muscle weakness.
What is the Pathology of Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-Like Episodes (MELAS)?
MELAS is a mitochondrial disease.
Etiology: MELAS is caused by mutations in mitochondrial DNA (mtDNA).
Genes involved: Mutations affecting the genes for mtDNA are inherited from the mother. MT-TL1, in MT-TQ, MT-TH, MT-TK, MT-TS1, MT-ND1, MT-ND5, MT-ND6, and MT-TS2.
Pathogenesis: Defective mitochondria.
Histology: The histology associated with Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS) shows diseased mitochondria proliferation in an attempt to compensate for poor energy production. They appear bright red compared to the blue myofibers, therefore called ragged red fibers.
How does Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-Like Episodes (MELAS) Present?
Patients with Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS) typically are both males and females equally. The disease begins in childhood between 2-15 years of age. The symptoms, features, and clinical findings associated with Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS) include seizures, headaches, hearing issues, ataxia, and myoclonus.
How is Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-Like Episodes (MELAS) Diagnosed?
Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS) is diagnosed based on clinical findings and molecular genetic testing. Muscle biopsy may show ragged red fibers.
How is Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-Like Episodes (MELAS) Treated?
Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS) has no specific treatment. Anticonvulsant medications may help with seizures.
What is the Prognosis of Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-Like Episodes (MELAS)?
The prognosis of Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS) is poor.
What is Myoclonic Epilepsy and Ragged Red Fibers (MERRF)?
Myoclonic epilepsy and ragged red fibers (MERRF) is an inherited mitochondrial disorder characterized by myoclonus epilepsy, ataxia, generalized seizures, and myopathy.
What is the Pathology of Myoclonic epilepsy and ragged red fibers (MERRF)
Etiology: The cause of Myoclonic epilepsy and ragged red fibers (MERRF) is mutations in the mitochondrial DNA.
Genes involved: Mutations in the MT-TK gene.
Pathogenesis: The sequence of events that lead to myoclonic epilepsy and ragged red fibers (MERRF) is pathogenic variants in mtDNA transmitted by maternal inheritance.
Histology: The histology associated with myoclonic epilepsy and ragged red fibers (MERRF) shows red ragged fibers and focal cytochrome c oxidase (CCO) deficiency. Along the length of single muscle fibers, defects in CCO activity were distributed segmentally with blurred borders.
How does Myoclonic Epilepsy and Ragged Red Fibers (MERRF) Present?
Patients with Myoclonic epilepsy and ragged red fibers (MERRF) have equal male and female predominance. The symptoms, features, and clinical findings associated with Myoclonic epilepsy and ragged red fibers (MERRF) include myoclonus, ataxia, optic atrophy, and epilepsy.
How is Myoclonic Epilepsy and Ragged Red Fibers (MERRF) Diagnosed?
Myoclonic epilepsy and ragged red fibers (MERRF) is diagnosed by medical history, symptoms, physical exam, and laboratory test results in order to make a diagnosis. Genetic testing is the main test of choice.
How is Myoclonic Epilepsy and Ragged Red Fibers (MERRF) Treated?
Myoclonic epilepsy and ragged red fibers (MERRF) has no cure.
What is the Prognosis of Myoclonic Epilepsy and Ragged Red Fibers (MERRF)?
The prognosis of myoclonic epilepsy and ragged red fibers (MERRF) is globally poor because of the progressive nature of the disease.
What are Tumors of the Central Nervous System?
Tumors of the central nervous system are abnormal cells in the nervous system which rapidly divide and have the ability to metastasize.
Examples of tumors of the central nervous system include:
- Gliomas
- Astrocytomas
- Oligodendrogliomas
- Ependymomas
- Periventricular mass lesions
- Neuronal tumors
- Poorly differentiated neoplasms of the central nervous system
What are Gliomas?
Gliomas originate in the glial cells that surround nerve cells. Three types of glial cells can produce tumors. Gliomas are classified according to the type of glial cell involved in the tumor, as well as the tumor’s genetic features, which can help predict how the tumor will behave over time and the treatments most likely to work.
Examples of gliomas include:
- Astrocytomas
- Oligodendrogliomas
- Ependymomas
- Paraventricular Mass Lesions
- Neuronal Tumors
What are Astrocytomas?
Astrocytomas originate in a particular kind of glial cells, star-shaped brain cells in the cerebrum called astrocytes. T
What is the Pathology of Astrocytomas?
Etiology: The cause of Astrocytomas is not known. Genetic and immunologic abnormalities, environmental factors, diet, stress, and/or other factors may play contributing roles in causing specific types of cancer.
Genes involved: Astrocytomas can have a genetic link when they are associated with a few rare, inherited disorders. These include neurofibromatosis type I, Li-Fraumeni syndrome, Turcot syndrome, and tuberous sclerosis.
Pathogenesis: The exact pathogenesis of astrocytoma is not completely understood but it is believed that this tumor has a close association with genetic mutations. The origin of this tumor is from neuroepithelial cells.
Histology: The histology associated with Astrocytomas shows diffuse astrocytomas with a mild increase in cellularity and fibrillary background.
How does Astrocytoma Present?
Patients with Astrocytomas typically are older males. The symptoms, features, and clinical findings associated with Astrocytomas include headaches, vision changes, seizures, and fatigue.
How is Astrocytoma Diagnosed?
Astrocytomas are diagnosed through clinical evaluation, characteristic physical findings, a careful patient history, and specialized tests, such as blood tests, neuroimaging techniques, and a biopsy.
How is Astrocytoma Treated?
Astrocytomas are treated by surgical resection with or without chemotherapy.
What is the Prognosis of Astrocytoma?
The prognosis of Astrocytoma fair.
What are Oligodendrogliomas?
Oligodendrogliomas is a tumor that develops from oligodendrocytes.
What is the Pathology of Oligodendrogliomas?
Etiology: The cause of Oligodendrogliomas is not known.
Genes involved: IDH mutation and 1p19q codeletion.
Pathogenesis: Expansion of clonal oligodedrycytes.
Histology: The histology associated with oligodendrogliomas shows regular cells with spherical nuclei containing finely granular chromatin surrounded by a halo of cytoplasm that is described as a “fried egg” appearance.
How does Oligodendrogliomas Present?
Patients with oligodendrogliomas are predominantly males, most often in people between the ages of 35 and 44. The symptoms, features, and clinical findings associated with Oligodendrogliomas include seizures, memory issues, headaches, and balance problems.
How is Oligodendroglioma Diagnosed?
Oligodendrogliomas are diagnosed by neurological exam, imaging, and biopsy.
How is Oligodendroglioma Treated?
Oligodendrogliomas can be treated by surgical resection with or without chemotherapy or radiation therapy.
What is the Prognosis of Oligodendrogliomas?
The prognosis of Oligodendrogliomas is fair.
What are Ependymomas?
Ependymomas are a very rare type of tumor that start in the brain or spinal cord. Ependymomas may occur in both children and adults.
What is the Pathology of Ependymomas?
Etiology: The cause of ependymomas is unknown.
Genes involved: Unknown. Increased risk with NF2.
Pathogenesis: Clonal expansion.
Histology: The histology associated with ependymomas shows well circumscribed tumors of 3 major types which include cellular (with clear cell and papillary), myxopapillary, and tanycytic.
How do Ependymomas Present?
Patients with ependymomas are typically males. Symptoms occur more commonly before 40 years of age. The symptoms, features, and clinical findings associated with Ependymomas include headaches, vomiting, dizziness, and seizures.
How is Ependymoma Diagnosed?
Ependymoma is diagnosed by neurological exam, imaging, and biopsy.
How is Ependymoma Treated?
Ependymomas are treated with surgical resection. Radiosurgery or chemotherapy may also be considered.
What is the Prognosis of Ependymomas?
The prognosis of Ependymoma is fair.
What are Periventricular Mass Lesions?
Periventricular mass lesions are a broad collection of pathological processes that result in changes on brain imaging. Periventricular mass lesions include a very disparate group of conditions ranging from infection (abscess) to brain tumors.
What is the Pathology of Periventricular Mass Lesions?
Etiology: The causes of periventricular mass lesions depends on the underlying cause which may include age related changes, infections, trauma, and tumors.
Pathogenesis: Age is certainly the most common association of periventricular mass lesions.
Histology: The histology associated with periventricular mass lesions shows microcysts filled with basophilic myxoid substance, clustering of nuclei, and finely fibrillary background. There is loose matrix and clusters of cells embedded in it.
How does Periventricular Mass Lesions Present?
Patients with periventricular mass lesions are typically males, and common above 50 years of age. The symptoms, features, and clinical findings associated with periventricular mass lesions include nausea, vomiting, headaches, visual disturbances, and loss of concentration.
How are Periventricular Mass Lesions Diagnosed?
Periventricular mass lesions are diagnosed by imaging.
How is Periventricular Mass Lesion Treated?
Periventricular mass lesion is treated by based on the underlying cause.
What is the Prognosis of Periventricular Mass Lesion?
The prognosis of periventricular mass lesions ranges from good to poor depending on what the underlying cause is.
What are Neuronal Tumors?
Neuronal tumors are tumors of the central nervous system that contain abnormal neuronal elements.
What is the Pathology of Neuronal Tumor?
Etiology: The causes of neuronal tumors include gene mutations, environmental toxins, and infections.
Pathogenesis: Age is certainly the most common association.
Histology: The histology associated with neuronal tumor shows mitotic figures, clusters of cells embedded in matrix, and increased cellularity.
How does Neuronal Tumor Present?
Patients with Neuronal Tumor are typically males and it is common above 40 years of age. The symptoms, features, and clinical findings associated with neuronal tumors include cranial feeling of pressure, headaches, nausea, visual changes, and fatigue.
How is Neuronal Tumor Diagnosed?
Neuronal tumor is diagnosed by by imaging and biopsy.
How is Neuronal Tumor Treated?
Neuronal tumors are treated by surgery with or without chemotherapy or radiotherapy.
What is the Prognosis of Neuronal Tumors?
The prognosis of neuronal tumors which are low grade is good. High grade tumors have worse prognosis.
What are Poorly Differentiated Neoplasms of the Central Nervous System?
Poorly differentiated neoplasms of the central nervous system are tumors of the CNS which are dangerous, aggressive, fatal and have a high metastatic potential.
Poorly differentiated neoplasms of the central nervous system include:
- Medulloblastoma
- Atypical teratoid tumor
- Atypical rhabdoid tumor
What is a Medulloblastoma?
A medulloblastoma is a common type of brain tumor.
What is the Pathology of Medulloblastoma?
Etiology: The cause of Medulloblastoma is not known.
Genes involved: PTCH2 on chromosome 1p32, CTNNB1 on chromosome 3p, and APC on chromosome 5q.
Pathogenesis: The sequence of events that lead to medulloblastoma arises from remnants of the primitive neuroectoderm in the roof of the fourth ventricle.
Histology: The histology associated with medulloblastoma is characterized by small cells with round to ovoid nuclei, Homer Wright rosettes, and no significant cytologic atypia or pleomorphism.
How does Medulloblastoma Present?
Patients with medulloblastoma typically are children of age four or younger. They can also be patients of age 5-14 years. Males are predominantly affected. The symptoms, features, and clinical findings associated with Medulloblastoma include headaches, nausea, and vomiting. Medulloblastoma is associated with certain inherited diseases, which include Li-Fraumeni syndrome, and nevoid basal cell carcinoma syndrome (Gorlin syndrome).
How is Medulloblastoma Diagnosed?
Medulloblastoma is diagnosed by biopsy.
How is Medulloblastoma Treated?
Medulloblastoma is treated primarily by surgery.
What is the Prognosis of Medulloblastoma?
The prognosis of Medulloblastoma is poor.
What is an Atypical Rhabdoid Tumor?
An Atypical Rhabdoid /Teratoid Tumor is a rare, fast-growing tumor of the brain and spinal cord. Atypical rhabdoid tumor usually occurs in children aged three years and younger, although it can occur in older children and adults. About half of these tumors form in the cerebellum or brain stem.
What is the Pathology of Atypical Rhabdoid Tumor?
Etiology: The cause of Atypical Rhabdoid /Teratoid Tumor is genetic.
Genes involved: SMARCB1 (also called INI1)con chromosome 22.
Pathogenesis: The sequence of events that lead to Atypical Rhabdoid /Teratoid Tumor occur mostly in the posterior cranial fossa and supratentorial region.
Histology: The histology associated with Atypical Rhabdoid /Teratoid Tumor shows jumbled small and large cells. The tissue of this tumor contains many different types of cells including the rhabdoid cells, large spindled cells, and epithelial and mesenchymal cells, and areas resembling primitive neuroectodermal tumor (PNET).
How does Atypical Rhabdoid Tumor Present?
Patients with Atypical Rhabdoid /Teratoid Tumor are mostly males, aged 3 years or younger. It can, however, occur in adults as well. The symptoms, features, and clinical findings associated with Atypical Rhabdoid /Teratoid Tumor include nausea, vomiting, and headache.
How is Atypical Rhabdoid Tumor Treated?
Atypical Rhabdoid /Teratoid Tumor is treated by surgery.
What is the Prognosis of Atypical Rhabdoid Tumor?
The prognosis of Atypical Rhabdoid /Teratoid Tumor is very poor.
What are Other Parenchymal Tumors?
Examples of other parenchymal tumors include:
- Primary CNS lymphoma
- Germ cell tumors
- Pineal parenchymal tumors.
What is a Primary CNS Lymphoma?
A primary CNS lymphoma is a rare form of extranodal non-Hodgkin lymphoma that is typically confined to the brain, eyes, and cerebrospinal fluid without evidence of systemic spread.
What is the Pathology of Primary CNS Lymphoma?
Etiology: The cause of Primary CNS Lymphoma is unknown. It is common in immunocompetent patients and elderly.
Genes involved: The failure of DNA double-strand breaks may lead to the formation of malignant cells.
Pathogenesis: Central nervous system is an immune privileged site and the lymphoma itself may have genetic and phenotypic features that protect the tumor from ongoing immune surveillance. Neoplastic cells may arise from lymphocytes normally residing in the immune privileged milieu of the CNS or from lymphocytes that transform to a malignant clone outside the CNS.
Histology: The histology associated with Primary CNS Lymphoma shows a vasocentric growth pattern and high lymphocyte proliferation, explaining its diffuse infiltration in the central nervous system (CNS).
How does Primary CNS Lymphoma Present?
Patients with Primary CNS Lymphoma are typically males and the common age group is above 45 years of age. The symptoms, features, and clinical findings associated with Primary CNS Lymphoma include nausea, vomiting, weight loss, and confusion.
How is Primary CNS Lymphoma Diagnosed?
Primary CNS Lymphoma is diagnosed by biopsy.
How is Primary CNS Lymphoma Treated?
Primary CNS Lymphoma is treated by high dose methotrexate.
What is the Prognosis of Primary CNS Lymphoma?
The prognosis of Primary CNS Lymphoma is poor.
What are Germ Cell Tumors?
A germ cell tumor is a neoplasm derived from germ cells which may be benign or malignant.
What is the Pathology of Germ Cell Tumors?
Etiology: The cause of Germ Cell Tumors is unknown.
Genes involved: Unknown.
Pathogenesis: Proliferation of germ cells.
Histology: The histology associated with Germ Cell Tumors shows resemblance to seminoma / dysgerminoma. There are epithelioid cells with abundant PAS+ cytoplasm, and large, round nuclei with irregularity and pleomorphism. They may have prominent nests of lymphocytes with occasional granulomatous inflammation that may obscure tumor cells. Frequent mitotic activity and necrosis may be observed.
How does Germ Cell Tumors Present?
Patients with Germ Cell Tumors typically are of age group 10-19 years and males are more commonly affected. The symptoms, features, and clinical findings associated with Germ Cell Tumors include thirst, excess urination, nausea, and vomiting.
How is Germ Cell Tumor Diagnosed?
Germ cell tumor is diagnosed by physical exam and biopsy.
How is Germ Cell Tumor Treated?
Germ cell tumors are treated by surgical resection with or without chemotherapy.
What is the Prognosis of Germ Cell Tumors?
The prognosis of Germ Cell Tumors is fair.
What is a Pineal Parenchymal Tumors?
A pineal parenchymal tumor is a primary central nervous system (CNS) tumor. These tumors begin in the brain (in the pineal gland) but can spread to the spinal cord.
What is the Pathology of Pineal Parenchymal Tumors?
Etiology: The cause of Pineal Parenchymal Tumors is unknown.
Genes involved: SYP (Synaptophysin).
Pathogenesis: Abnormal proliferation of pineal cells.
Histology: The histology associated with Pineal Parenchymal Tumors shows 2 distinct morphologic subtypes: large-cell and small-cell. H&E stained sections of the large-cell subtype show larger nuclei with greater nuclear pleomorphism, more abundant cytoplasm, and more abundant neurophil-like stroma in the background. H&E stained sections of the small-cell subtype show smaller, more uniform nuclei with inconspicuous nucleoli and scant cytoplasm with very little stroma in the background.
How does Pineal Parenchymal Tumors Present?
Patients with Pineal Parenchymal Tumors are typically males. The common age group is 20-64 years. The symptoms, features, and clinical findings associated with Pineal Parenchymal Tumors include headaches, nausea, and vomiting.
How is Pineal Parenchymal Tumor Diagnosed?
Pineal Parenchymal Tumor is diagnosed by biopsy.
How is Pineal Parenchymal Tumor Treated?
Pineocytomas are treated with surgical resection. If complete or subtotal resection is accomplished, the outcome is favorable, even without adjuvant treatment.
What is the Prognosis of Pineal Parenchymal Tumors?
The prognosis of Pineal Parenchymal Tumor is poor, but can get better with treatment.
What are Meningiomas?
Meningiomas are the most common type of primary brain tumors. These tumors originate in the meninges, which are the outer three layers of tissue between the skull and the brain that cover and protect the brain just under the skull.
What is the Pathology of Meningioma?
Etiology: The causes of meningioma include radiation, hormone replacement therapy, and germ line mutations.
Genes involved: Monosomy 22, NF2, TRAF7, AKT1, KLF4, SMO, and PIK3CA.
Pathogenesis: Meningiomas driven by chromosome proliferation of atypical cells.
Histology: The histology associated with Meningioma shows epithelioid cells with round to oval nuclei and streaked cytoplasm. It may contain intranuclear pseudoinclusions. Meningothelial nests or whorls and psammoma bodies.
How does Meningioma Present?
Patients with Meningioma typically are adult females, with age greater than 65 years. The symptoms, features, and clinical findings associated with Meningioma include vision changes, head aches, and weakness.
How is Meningioma Diagnosed?
Meningioma is diagnosed by biopsy.
How is Meningioma Treated?
Meningioma is treated with surgery.
What is the Prognosis of Meningiomas?
The prognosis of Meningiomas is fair.
What are Familial Tumor Syndromes?
Familial tumor syndromes are genetic disorders in which inherited genetic mutations in one or more genes predispose the affected individuals to the development of certain cancers,
Examples of familial tumor syndromes include:
- Tuberous sclerosis
- Von Hippel-Lindau disease
- Neurofibromatosis
What is Tuberous Sclerosis?
Tuberous sclerosis is a rare genetic multisystem disorder with a wide range of potential signs and symptoms and is associated with the formation of benign tumors.
What is the Pathology of Tuberous sclerosis?
Etiology: The cause of Tuberous sclerosis is mutation in one of two different genes, the TSC1 gene or the TSC2 gene.
Genes involved: TSC1 and TSC2.
Pathogenesis: In the cerebrum of patients with tuberous sclerosis (TSC), there are three types of nodular lesions: cortical tubers, subcortical heterotopic nodules and subependymal giant cell astrocytomas.
Histology: The histology associated with Tuberous sclerosis shows hamartomas which contain abnormal giant cells that show evidence of abnormal differentiation of immature neural cells.
How does Tuberous Sclerosis Present?
Patients with Tuberous sclerosis typically are males and females with equal occurrence. The age range is 15-35 years old. The symptoms, features, and clinical findings associated with Tuberous sclerosis include seizures, muscle issues, developmental delays, and rhabdomyomas.
How is Tuberous Sclerosis Diagnosed?
Tuberous sclerosis is diagnosed based with clinical exam in combination with computed tomography (CT) or magnetic resonance imaging (MRI) of the brain. Biopsy may be helpful. —which may show tubers in the brain, and an ultrasound of the heart, liver, and kidneys, which may show tumors in those organs.
How is Tuberous Sclerosis Treated?
Tuberous sclerosis is treated with everolimus.
What is the Prognosis of Tuberous Sclerosis?
The prognosis of Tuberous sclerosis is poor.
What is Von Hippel-Lindau Disease?
Von Hippel-Lindau disease is an inherited disorder characterized by the formation of tumors and fluid-filled sacs (cysts) in many different parts of the body. These tumors may be either noncancerous or cancerous and most frequently appear during young adulthood.
What is the Pathology of Von Hippel-Lindau Disease?
Etiology: The cause of Von Hippel-Lindau disease is mutations in the VHL gene.
Genes involved: Mutations in the VHL gene.
Pathogenesis: Defects in tumor suppressors.
Histology: Von Hippel-Lindau disease histology shows hemangioblastoma, which may present with mesenchymal (upper) and epithelioid structures (lower). There are two main cellular constituents of hemangioblastomas; stromal cells and vascular cells.
How does Von Hippel-Lindau Disease Present?
Patients with Von Hippel-Lindau disease are typically 26 years of age on average. Males are more affected than females. The symptoms, features, and clinical findings associated with Von Hippel-Lindau disease include headaches, vision problems, and balance issues.
How is Von Hippel-Lindau Disease Diagnosed?
Von Hippel-Lindau disease is diagnosed with genetic testing.
How is Von Hippel-Lindau Disease Treated?
Von Hippel-Lindau disease is treated by surgery if needed, and medical management.
What is the Prognosis of Von Hippel-Lindau Disease?
The prognosis of Von Hippel-Lindau disease is poor.
What is Neurofibromatosis?
Neurofibromatosis is a group of genetic disorders that cause tumors to form on nerve tissue.
What is the Pathology of Neurofibromatosis?
Etiology: The cause of Neurofibromatosis is a family history of the disorder.
Genes involved: NF1 and NF2.
Pathogenesis: The sequence of events that lead to Neurofibromatosis is caused by genetic defects.
Histology: The histology associated with Neurofibromatosis shows proliferation of all elements of peripheral nerves. Schwann cells with wire-like collagen fibrils, mast cells, and stromal mucosubstance.
How does Neurofibromatosis Present?
Patients with Neurofibromatosis typically are children of no more than 10 years of age. The symptoms, features, and clinical findings associated with Neurofibromatosis include cafe au lait spots, Lisch nodules, optic gliomas, and hearing issues.
How is Neurofibromatosis Diagnosed?
Neurofibromatosis is diagnosed by physical exam, and biopsy. Genetic tests for SMARCB1 and LZTR1 may be helpful.
How is Neurofibromatosis Treated?
Neurofibromatosis is treated by symptomatic management and prevention.
What is the Prognosis of Neurofibromatosis?
The prognosis of Neurofibromatosis is good.
What are Other Syndromes that Have Malignancies that Effect the Central Nervous System?
Others syndromes that have malignancies that affect the central nervous system include:
- Cowden syndrome
- Gorlin syndrome
- Li-Fraumeni syndrome
- Turcot syndrome
What is Cowden Syndrome?
Cowden syndrome is a genetic disorder characterized by multiple noncancerous, tumor-like growths called hamartomas and an increased risk of developing certain cancers. Almost everyone with Cowden syndrome develops hamartomas.
What is the Cowden Syndrome?
Etiology: The cause of Cowden syndrome mutations in a gene known as PTEN.
Genes involved: PTEN.
Pathogenesis: PTEN acts as a tumor suppressor gene through the action of it’s phosphatase protein product. This phosphatase is involved in the regulation of the cell cycle, preventing cells from growing and dividing too rapidly. It is a target of many anticancer drugs.
Histology: The histology associated with Cowden syndrome shows glycogenic acanthosis, ganglioneuroma, and hamartomatous polyp.
How does Cowden Syndrome Present?
Patients with Cowden syndrome are typically more females than males, and symptoms appear by the age of 20 years old. The symptoms, features, and clinical findings associated with Cowden syndrome include macrocephaly, intellectual disability, and skin lesions.
How is Cowden Syndrome Diagnosed?
Cowden syndrome is diagnosed by a diagnostic criteria.
Major criteria:
- Breast cancer
- Endometrial cancer
- Thyroid carcinoma
- Gastrointestinal hamartomas excluding hyperplastic polyps >3
- Lhermitte-Duclos disease
- Macrocephaly (58cm females, 60cm males)
- Macular pigmentation of the glans penis
- Multiple mucocutaneous lesions
- Multiple trichilemmomas >3
- Acral keratosis >3
- Mucocutaneous neuromas >3
- Oral papillomas, multiple >3, OR biopsy proven, or dermatologist diagnosed
Minor criteria:
- Autism spectrum disorder
- Colon cancer
- Esophegal glycogenic acanthosis
- Lipomas >3
- Mental retardation <75 IQ
- Renal cell carcinoma
- Testicular lipomatosis
- Thyroid cancer
- Thyroid structural lesions
- Vascular anomalies
- CT and MRI can also be used for diagnosis
How is Cowden Syndrome Treated?
Cowden syndrome is treated in the same fashion as those that occur sporadically in patients without a hereditary cancer syndrome. The benign mucocutaneous lesions observed in Cowden syndrome are typically not treated unless they become symptomatic or disfiguring. Numerous treatment options include topical agents, cryosurgery, curettage, laser ablation, and excision, may be utilized.
What is the Prognosis of Cowden Syndrome?
The prognosis of Cowden syndrome is poor and there is no cure.
What is Gorlin Syndrome?
Gorlin syndrome is also known as nevoid basal cell carcinoma syndrome. Gorlin syndrome is a condition that affects many areas of the body and increases the risk of developing various cancerous and noncancerous tumors.
What is the Pathology of Gorlin Syndrome?
Etiology: The cause of Gorlin Syndrome is a mutation in the PTCH1 gene. Nevoid basal cell carcinoma syndrome (NBCCS) is inherited in an autosomal dominant pattern. All individuals inherit two copies of each gene.
Genes involved: (PTCH1), a tumor suppressor gene located on chromosome 9q. Mutations in suppressor of fused gene (SUFU) on chromosome 10q and PTCH2 on chromosome 1p
Pathogenesis: The sequence of events that lead to Gorlin Syndrome Gorlin syndrome a mutation in patched 1 (PTCH1), a tumor suppressor gene located on chromosome 9q. The PTCH1 gene encodes a transmembrane receptor protein that recognizes signaling proteins of the sonic hedgehog family. Homozygous inactivation of the PTCH gene leads to tumorigenecity and the formation of multiple basal cell carcinomas and other tumors.
Histology: The histology associated with Gorlin Syndrome shows tumor nests consisting of basiloid cells having cellular and nuclear atypia and arranged in a palisading fashion, dispersed throughout the dermis.
How does Gorlin Syndrome Present?
Patients with Gorlin Syndrome are more males than females. Cancers start to develop around the age of 30. The symptoms, features, and clinical findings associated with Gorlin Syndrome include cerebral calcifications, medulloblastomas, macrocephaly, and multiple basal cell carcinomas.
How is Gorlin Syndrome Diagnosed?
Gorlin Syndrome is diagnosed clinically. Genetic testing can be done to help with the diagnosis.
How is Gorlin Syndrome Treated?
Gorlin Syndrome is treated by surgery, chemotherapy and radiotherapy.
What is the Prognosis of Gorlin Syndrome?
The prognosis of Gorlin Syndrome poor. There is no cure.
What is Li-Fraumeni Syndrome?
Li-Fraumeni syndrome is an inherited familial predisposition to a wide range of certain, often rare, cancers. This is due to a change (mutation) in a tumor suppressor gene known as TP53. The resulting p53 protein produced by the gene is damaged and is unable to help prevent malignant tumors from developing.
What is the Pathology of Li-Fraumeni Syndrome?
Etiology: The cause of Li-Fraumeni syndrome is mutation of the TP53 tumor suppressor gene on chromosome 17. LFS follows autosomal dominant inheritance.
Genes involved: TP53 tumor suppressor gene on chromosome 17.
Pathogenesis: The sequence of events that lead to Li-Fraumeni syndrome is germline TP53 gene mutation.
Histology: The histology associated with Li-Fraumeni syndrome is nonspecific.
How does Li-Fraumeni Syndrome Present?
Patients with Li-Fraumeni syndrome typically are females and usually present at the age of 40 years. The symptoms, features, and clinical findings associated with Li-Fraumeni syndrome include multiple cancers such as soft tissue sarcomas, osteosarcomas, leukemia, and gonadal tumors.
How is Li-Fraumeni Syndrome diagnosed?
Li-Fraumeni syndrome is diagnosed based on the presence of a so called pathogenic or likely pathogenic variant in the TP53 gene.
How is Li-Fraumeni Syndrome Treated?
There is no standard treatment or cure for LFS or a germline TP53 gene variant. continues on how to best manage those cancers involved in LFS. This includes surgery, chemotherapy, and radiotherapy.
What is the Prognosis of Li-Fraumeni syndrome?
The prognosis of Li-Fraumeni syndrome poor.
What is Turcot Syndrome?
Turcot syndrome is a rare inherited disorder characterized by the association of benign growths (adenomatous polyps) in the mucous lining of the gastrointestinal tract with tumors of the central nervous system.
What is the Pathology of Turcot Syndrome?
Etiology: The cause of Turcot syndrome is unknown.
Genes involved: APC mutation is considered important.
Pathogenesis: Turcot syndrome is inherited as an autosomal recessive trait and the other as an autosomal dominant trait.
Histology: The histology associated with Turcot syndrome shows high grade neoplasms, with significant mass effect. Palisaded necrosis is also often observed.
How does Turcot Syndrome Present?
Patients with Turcot syndrome typically have a family history of multiple malignancies.
The symptoms, features, and clinical findings associated with Turcot syndrome include multiple benign growths (polyps) in the colon, rectal bleeding, gliomas, and lipomas.
How is Turcot Syndrome Diagnosed?
Turcot syndrome is diagnosed based upon a detailed patient history, a thorough clinical evaluation, and a variety of specialized tests. Genetic testing for APC mutations may be indicated.
How is Turcot Syndrome Treated?
The treatment of Turcot syndrome is directed toward the specific symptoms that are apparent in each individual. Surgical removal of the tumors may be indicated.
What is the Prognosis of Turcot Syndrome?
The prognosis of Turcot syndrome is fair with a median life expectancy of 45 years.
What are Paraneoplastic Syndromes that Affect the Central Nervous System?
Paraneoplastic syndromes that affect the central nervous system are rare disorders that are triggered by an altered immune system response to a neoplasm. They are defined as clinical syndromes involving nonmetastatic systemic effects that accompany malignant disease.
Paraneoplastic syndromes that affect the central nervous system include:
- Eye movement disorders
- Lambert-Eaton myasthenic syndrome
- Subacute cerebellar degeneration
- Limbic encephalitis
What are Eye Movement Disorders?
Eye movement disorders are prominent features of a range of paraneoplastic syndromes, especially those involving the brainstem and cerebellum.
What is the Pathology of Eye Movement Disorders?
Etiology: The cause of Eye movement disorders is from immune mechanisms of the body directed against tumor related antigens. The host produces antibodies against the cancer antigen, resulting in an autoimmunization and then autoantibodies against normal host tissue.
Pathogenesis: The sequence of events that lead to Eye movement disorders is that immunological responses to neuronal antigens expressed by the underlying cancer are also active against receptors or channels on neurons.
How do Eye Movement Disorders Present?
Patients with eye movement disorders typically present with visual disturbances.
How are Eye Movement Disorders Diagnosed?
The diagnosis of the eye movement disorders depends on the clinical presentation, the ophthalmologic findings and finally the identification of the offending antibodies.
How are Eye Movement Disorders Treated?
Eye movement disorders associated with paraneoplastic syndromes are treated by treating the underlying malignancy.
What is the Prognosis of Eye Movement Disorders?
The prognosis of eye movement disorders is poor.
What is Lambert-Eaton Myasthenic Syndrome?
Lambert-Eaton myasthenic syndrome is a rare autoimmune disorder of the neuromuscular junction.
What is the Pathology of Lambert-Eaton Myasthenic Syndrome?
Etiology: The cause of Lambert-Eaton myasthenic syndrome is often a certain type of cancer called small cell lung cancer.
Pathogenesis: The sequence of events that lead to Lambert-Eaton myasthenic syndrome involves antibodies against the voltage-gated calcium channels that mediate the release of acetylcholine which blocks the normal flow of calcium. This prevents the release of acetylcholine from its vesicles. There is little to no acetylcholine entering the synaptic cleft. Therefore, there will be minimal to no muscle contraction.
How does Lambert-Eaton Myasthenic Syndrome Present?
Patients with Lambert-Eaton myasthenic syndrome are usually males over the age of 50 years old. The symptoms, features, and clinical findings associated with Lambert-Eaton myasthenic syndrome include weakness, respiratory issues, hyporeflexia, and autonomic dysfunction.
How is Lambert-Eaton Myasthenic Syndrome Diagnosed?
Lambert-Eaton myasthenic syndrome is diagnosed via electrophysiological testing.
How is Lambert-Eaton Myasthenic Syndrome Treated?
Lambert-Eaton myasthenic syndrome has no cure. Treatments include potassium channel blockers such as guanidine and aminopyridine. Acetylcholinesterase inhibitors like pyridostigmine may help prevent the destruction of acetylcholine. Chemotherapy regimens target the associated tumor.
What is the Prognosis of Lambert-Eaton Myasthenic Syndrome?
The prognosis of Lambert-Eaton myasthenic syndrome is poor.
What is Subacute Cerebellar Degeneration?
Subacute cerebellar degeneration is characterized by the deterioration of the area of the brain concerned with muscle coordination and balance.
There are two types of subacute cerebellar degeneration:
- Paraneoplastic cerebellar degeneration due to cancer
- Thiamine deficiency associated cerebellar degeneration, caused by a lack of the vitamin B1 (thiamine) which may be related to alcoholism or a poor diet
What is the Pathology of Subacute Cerebellar Degeneration?
Etiology: The cause of Subacute Cerebellar Degeneration is autoimmune.
Pathogenesis: Autoimmune mediated antibodies and T-cells attacking normal cells in the nervous systems.
How does Subacute Cerebellar Degeneration Present?
Patients with subacute cerebellar degeneration are typically over 50 years of age on average, and females are predominantly affected. The symptoms, features, and clinical findings associated with Subacute Cerebellar Degeneration include ataxia, dysarthria, dysphagia, diplopia, tremors, and vertigo.
How is Subacute Cerebellar Degeneration Diagnosed?
The diagnostic criteria for paraneoplastic subacute cerebellar degeneration are:
- Severe cerebellar symptoms less than 12 weeks, along with no abnormal sign of cerebellar size reduction
- Rankin scale score of at least a 3, indicating that symptoms interfere with daily life significantly
- Clinical evidence of cerebellar dysfunction, specifically within the trunk or hemispheres, and established diagnosis of cancer within 5 years from the onset of symptoms
- Cerebellar symptoms and cancer-related antibodies
How is Subacute Cerebellar Degeneration Treated?
Subacute cerebellar degeneration is treated by prompt tumor removal, chemotherapy and radiation therapy. Adjuvant therapy with glucocorticoids such as methylprednisolone and immunotherapy with potent T cell inhibition, such as tacrolimus and rituximab may be helpful. Physical therapy and ocupational therapy should aso be considered.
What is the Prognosis of Subacute Cerebellar Degeneration?
The prognosis of subacute cerebellar degeneration is poor. Most symptoms (such as ataxia and vision problems) can be reversed if detected and treated promptly. However, improvement in memory function and cognitive skills may be slow and, usually, incomplete. Without treatment, this condition can be disabling and life-threatening.
What is Limbic Encephalitis?
Limbic encephalitis is a form of autoimmune encephalitis that is characterized by inflammation of the brain.
What is the Pathology of Limbic Encephalitis?
Etiology: The cause of Limbic Encephalitis with an autoimmune reaction.
Pathogenesis: The pathological changes of paraneoplastic limbic encephalitis are usually related to loss of neurons with reactive gliosis, perivascular lymphocytic cuffing, and microglial proliferation.
Histology: The histology associated with Limbic Encephalitis shows multiple areas of micro-softening in the cerebral cortex which are distributed in the limbic and non-limbic areas with lymphocytic infiltration in the intraparenchymal vessels.
How does Limbic Encephalitis Present?
Patients with limbic encephalitis are predominantly males and age range is 40-45 years. The symptoms, features, and clinical findings associated with Limbic Encephalitis include personality changes, depression, irritability, memory loss, and seizures.
How is Limbic Encephalitis Diagnosed?
Limbic Encephalitis is diagnosed by clinical exam, and blood tests.
How is Limbic Encephalitis Treated?
Limbic Encephalitis is treated by surgical removal of any tumors that mey be present, chemotherapy if indicated, and supportive care.
What is the Prognosis of Limbic Encephalitis?
The prognosis of limbic encephalitis is fair.
What are Metastatic Tumors?
Metastatic tumors are tumors that spread to other parts of the body. Metastatic tumors that affect the central nervous system are typically carcinomas, but may originate from many different malignancies.