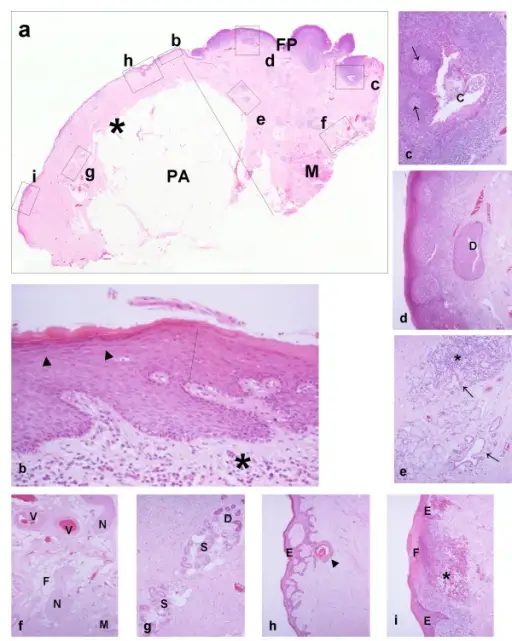
Inflammation is a response to injury or infection in order to defend the body from viruses, bacteria, or perceived immunologic threats. Inflammation usually consists of two phases; the acute phase, and the chronic phase. The inflammatory process involves the activation and migration of leukocytes from blood vessels into tissues, where they release chemical mediators that cause tissue damage. These inflammatory chemicals include histamine, serotonin, bradykinin, prostaglandins, platelet activating factor and cytokines such as tumor necrosis factors alpha. In addition, these cells produce enzymes which degrade connective tissue proteins in order to allow for movement through damaged areas. This degradation leads to swelling and pain.
The term “inflammation” refers to an array of complex biological processes involving interactions between various cell populations and mediators of inflammation. The primary goal of these reactions is to remove harmful stimuli and initiate repair mechanisms. These events occur simultaneously throughout the entire organism; however, they have distinct temporal characteristics depending on the location and severity of the insult. For example, local inflammation involves recruitment of circulating white blood cells while systemic inflammation involves activation of multiple organ systems.
What Are the Causes of Inflammation?
Causes of inflammation are varied; however, most involve some type of physical trauma or an infectious agent. Other causes may be due to autoimmune disorders, allergies, tumors, radiation exposure, or toxic substances.
What is the Inflammatory Processes?
The inflammatory process can occur at any site within the body but usually occurs in two ways—acutely following traumatic events and chronically after repeated insults. Acute inflammation results when there is direct contact between irritants and living tissue. Chronic inflammation develops over time with repetitive stimuli. Both acute and chronic inflammations result in similar symptoms including redness, heat, swelling, tenderness, loss of function, and sometimes bleeding.
Acute inflammation begins immediately upon stimulation by foreign agents and lasts only 24-48 hours. It is characterized by increased vascular permeability, vasodilation, edema formation, exudate accumulation, neutrophil infiltration, and plasma extravasation. Neutrophils migrate toward sites of infection and phagocytize bacteria and other pathogens. They also secrete proteolytic enzymes that destroy invading microorganisms. Macrophages engulf debris and remove dead cells. Mast cells degranulate releasing histamines and heparin, causing local vasodilation and increasing capillary permeability. Platelets adhere to endothelial surfaces forming aggregates called thrombi. Thrombin activates coagulation cascade resulting in fibrin clot formation.
How Do Microbes Cause Inflammation?
Microbes cause inflammation by producing toxins that stimulate macrophage production of proinflammatory cytokines. Microbial products activate complement system leading to chemotaxis of polymorphonuclear leukocytes. Neutrophils then kill microbes via oxidative burst and lyse them using their granules containing antimicrobial peptides. When microbial cell walls become disrupted during pathogen invasion, host immune responses initiate signaling cascades that lead to expression of genes encoding various cytokines and growth factors. Cytokine synthesis induces recruitment of additional immune effector cells. Tissue destruction caused by activated macrophages releases endogenous danger signals, further stimulating innate immunity. Damage-associated molecules released from injured epithelium induce dendritic cells to mature and present antigens to lymphocytes. Activated lymphocytes proliferate and differentiate into cytotoxic T cells and memory B cells. Memory B cells form germinal centers where somatic hypermutation and affinity maturation take place. Antibodies produced by B cells bind antigenic targets on infected cells and facilitate clearance of pathogens.
How Do Damaged Cells Cause Inflammation?
Damaged cells cause inflammation by activating pattern recognition receptors expressed on resident immune cells such as monocyte/macrophages, mast cells, NK cells, DCs, basophils, eosinophils, platelets, keratinocytes, fibroblasts, etc. PRR activation leads to secretion of a variety of mediators involved in initiation and propagation of an inflammatory response.
What is Acute Inflammation?
Acute inflammation is a response to an injury or infection. It can be caused by physical, chemical and biological agents that damage the body’s tissues. Acute inflammatory responses are characterized by redness, swelling and heat.
What Are the Stages of Acute Inflammation?
Acute inflammation has three major stages: initiation, amplification, and termination.
- Initiation is characterized by immediate changes in gene transcription and synthesis of proteins required for migration of leukocytes to sites of injury.
- Amplification is marked by increased numbers of migrating leukocytes and their subsequent interaction with resident cells.
- Termination is associated with return to homeostasis through elimination of damaging stimuli and completion of wound repair. During this period, several different subsets of activated lymphoid cells migrate to affected organs where they participate in specific functions including destruction of infected/injured cells, promotion of angiogenesis, regulation of adaptive immune system development, and modulation of humoral immune responses.
Acute inflammation is usually self limiting within 48 hrs after initial insult. The acute phase involves rapid migration of white blood cells to site of injury followed by release of reactive oxygen species, lysosomal enzyme cathepsins, and matrix metalloproteinases. These processes are mediated through NFkB pathway.
What is the Vascular Response to Inflammation?
The vascular response to inflammation includes vasoconstriction, vasodilation and angiogenesis.
- Vasoconstrictive effects include decreased flow rate due to reduced cardiac output, decrease in arterial pressure, increase in peripheral resistance, constriction of venous capacitance vessels, and reduction in renal perfusion.
- Vasodilatory effects include increased flow rates due to enhanced myogenic activity, dilatation of large arteries, and relaxation of small veins.
- Angiogenesis occurs when tissue hypoxia stimulates VEGF production by perivascular stroma. This results in proliferation of pre-existing capillaries and sprouting of new ones.
What is the Lymphatic Response to Inflammation?
The lymphatic response to inflammation includes edema formation, accumulation of fluid in interstitial spaces, and enlargement of regional lymph nodes. Edema forms because of leakage of plasma proteins across endothelial barrier. Fluid accumulates in tissues because of osmotic forces generated by water movement between intravascular space and extracellular fluids. Lymph node swelling is due to expansion of medullary sinuses. Medullary sinus volume increases up to 10 fold following stimulation with bacterial lipopolysaccharide. Swelling begins at 2 hours post injection and peaks around 6 hours. It returns to baseline levels by 24 hours.
How Does Inflammation Result in Leukocyte Recruitment?
Leukocyte recruitment in inflammation involves a complex series of interactions among leukocytes, platelets, endothelium, chemokine receptors on the surface of these cells, adhesion molecules expressed on their surfaces, as well as matrix metalloproteinases secreted from them.
Leukocytes are recruited to sites of injury through two major mechanisms; rolling followed by firm arrest mediated primarily by selectins. Selectins bind carbohydrate ligands present on activated endothelial cell membranes.
The three known selectins are E-, P- and L-selectin. They have different affinities for carbohydrates and therefore recognize distinct glycoproteins. For example, E-selectin binds sialyl Lewis x structures while P-selectin recognizes sulfated glycans such as Slex/Slea. These selectins play an important role in inflammatory processes.
Rolling is initiated by binding of selectins to their respective ligand. Once bound, leukocytes roll along the vessel wall via interaction of integrins on their surface with ECM proteins. Integrin αIIbβ3 mediates this process.
After initial attachment, leukocytes become firmly adherent to the endothelium. Adherence requires activation of β1 integrins which interact with ICAM-1 and VCAM-1. Activation of β2 integrins leads to degranulation of granules containing proteolytic enzymes.
What Role Does Phagocytosis Have in Inflammation?
Phagocytosis in inflammation occurs when macrophages ingest foreign particles or damaged host tissue. Macrophage phagocytosis plays a key role in innate immunity against invading pathogens. Phagocytosis initiates signal transduction pathways leading to expression of genes involved in pathogen killing and clearance. This process may lead to release of proinflammatory factors that recruit additional immune effectors. Alternatively, ingestion of apoptotic cells results in downregulation of antigen presentation and generation of anti-inflammatory signals.
What Are the Cells of Acute Inflammation?
In general, neutrophils aka polymorphonuclear cells (PMNs) are the cells of acute inflammation. Neutrophils migrate through the endothelium and accumulate at sites of inflammation.
What Are the Mediators of Inflammation?
Mediators of inflammation include cytokines, chemotactic peptides, complement components, lipids, prostaglandins, and platelets. Cytokines are soluble polypeptides produced by many cell types following stimulation by agents such as bacterial lipopolysaccharide or cytokines themselves. Chemotactic peptides attract neutrophils and mononuclear cells to inflamed tissues. Complement components activate complement cascade resulting in opsonization of bacteria and induction of other cellular responses. Lipids released during arachidonate metabolism act locally at the site of production and also serve as precursors for eicosanoids. Prostaglandins induce vasodilation and increase vascular permeability. Histamine causes smooth muscle contraction and increases capillary permeability. Platelet activating factor induces aggregation of blood platelets and stimulates secretion of coagulating substances.
What Are the Morphologic Patterns of Acute Inflammation?
Morphologic patterns of acute inflammation include exudative, proliferative, and fibrous phases.
- Exudative phase begins immediately after insult and lasts until resolution of inflammation. It includes infiltration of polymorphonuclears into injured area, accumulation of fluid within interstitial spaces, and extravasation of plasma protein into surrounding connective tissue.
- Proliferative phase follows exudative phase and consists of proliferation of epithelial cells lining mucosal surfaces, formation of new vessels, and deposition of collagen fibers around areas of necrosis.
- Fibrotic phase represents final stage of healing and it can be observed weeks later. In chronic inflammation, there is persistent presence of infiltrating leukocytes and excessive extracellular matrix deposition. Chronic inflammation usually develops over time due to repeated exposure to irritants.
How Is the Acute Inflammatory Response Terminated?
Termination of acute inflammatory response depends on removal of injurious agent from body. If no further damage ensues, then acute inflammation resolves spontaneously with restoration of normal function. However, if injury persists, then chronic inflammation will develop.
What Are the Outcomes of Acute Inflammation?
Outcomes of acute inflammation depend upon the type of stimulus, intensity of initial insult, duration of insult, and genetic predisposition.
What is Chronic Inflammation?
Chronic inflammation is characterized by persistence of activated leukocytes in affected organ. The most common cause of chronic inflammation is infection.
What Causes Chronic Inflammation?
Causes of chronic inflammation may vary depending on underlying disease process. Some examples include infections, allergic disorders, autoimmunity, malignancy, and others. Other important etiological factors include trauma, radiation therapy and drug reactions.
Chronic inflammation is initiated by persistent irritation or infections. Repeated exposures to noxious stimuli eventually trigger the development of chronic disease states. For example, cigarette smoke contains more than 4,000 different compounds, many of which have been shown to contribute to lung diseases like emphysema and bronchitis.
What Are the Morphologic Features of Chronic Inflammation?
The morphologic features of chronic inflammation are similar to those seen in acute inflammation except that they tend to persist longer than a few days. They consist of increased number of macrophages, lymphoid follicles, granulomas, giant cell reaction, and foreign-body giant cell reaction. These morphological changes occur because of continued stimulation of immune system by antigen or its products.
What Are the Cells of Chronic Inflammation?
In general, the cells of chronic inflammation are lymphocytes. Lymphocytes infiltrate inflamed tissues where they interact with dendritic cells and activate them for presentation of antigens to naive T cells. Dendritic cells mature and differentiate into professional APCs. Antigen presenting cells take up apoptotic bodies containing self proteins released during cellular death and cross-present these antigens to naïve T cells. Thus, adaptive immunity is initiated.
What Are the Mediators of Chronic Inflammation?
Mediators of chronic inflammation can be divided into two groups based on whether they promote tissue destruction or repair.
Destructive mediators include matrix metalloproteinase enzymes, reactive oxygen species, nitric oxide synthase, arachidonic acid metabolites, platelet activating factor, and complement components.
Repair mediators include growth factors, chemokines, interleukins, transforming growth factor beta, and prostaglandin E2.
Activated T helper 1 cells produce cytokines such as IL-2, IFNγ, and TNFα which stimulate monocytic phagocytes to release reactive oxygen species and proteases. Th17 cells also play an important role in this process. B cells secrete antibodies against antigens presented by APCs. Macrophages present processed peptides derived from dead pathogens to CD4+T cells via MHC class II molecules. This results in activation of cytotoxic T cells. Mast cells degranulate and release histamines and other mediators causing local edema.
What Is Granulomatous Inflammation?
Granulomatous inflammation is defined as accumulation of epithelial cells around necrotic debris. It occurs when there is excessive production of inflammatory response leading to formation of granulation tissue. In general, granulomas consists of three layers namely outermost layer called Langhans’s multinucleate giant cell, middle zone consisting of fibroblasts and collagen fibers, and innermost area composed of epithelioid cells.
The main causes of granulomatous inflammation are tuberculosis, sarcoidosis, Crohn’s disease, Wegener’s granulomatosis, fungal diseases like candidiasis, cryptococcosis, blastomycosis etc., parasitic infestations, autoimmune conditions like rheumatoid arthritis, systemic lupus erythematosus, vasculitis, graft versus host disease, hypersensitivity pneumonitis, idiopathic pulmonary fibrosis, alveolar proteinosis, eosinophilic pneumonia, bronchiolitis obliterans organizing pneumonia, amiodarone induced lung injury, inhalational exposure to toxic fumes, drugs including antibiotics, antihypertensive agents, nonsteroidal antiinflammatory drugs, chemotherapy, radiotherapy, and others.
What Are the Systemic Effects of Inflammation?
The systemic effects of inflammation include fever, malaise, loss of appetite, weight loss, fatigue, muscle aches, joint pains, headache, nausea, vomiting, diarrhea, abdominal pain, cough, dyspnea, wheezing, skin rash, swelling, red eyes, sore throat, swollen lymph nodes, chest tightness, coughing up blood, difficulty breathing, dizziness, fainting, heart palpitations, confusion, seizures, coma, shock, kidney failure, liver damage, bleeding disorders, increased risk of infection, decreased immune function, and even death. These symptoms may occur suddenly or gradually over a period of days or weeks.
The severity of illness depends upon how much tissue has been damaged by the initial insult, the intensity of the subsequent inflammatory reaction, and the duration of the inflammatory state. In some cases, the body attempts to control the extent of the inflammatory response by producing endogenous cortisone that suppresses it. However, if the inflammatory stimulus persists, then the body will not have enough cortisone available to suppress the ongoing inflammatory response. As a result, the patient may become increasingly ill.
What Are Some Inflammation Associated Diseases?
Inflammation associated diseases include asthma, arthritis, atherosclerosis, cancer, chronic obstructive pulmonary disease, cystic fibrosis, diabetes mellitus, gout, glomerulonephritis, multiple sclerosis, obesity, osteoarthritis, psoriasis, rheumatoid arthritis, septic shock syndrome, systemic lupus erythematosus, and ulcerative colitis.