The role of apoptosis in immunologic tolerance is the deletion of T cells expressing self-antigens and T-cells infected with viruses.
What is the Role of Apoptosis in Immunologic Tolerance?
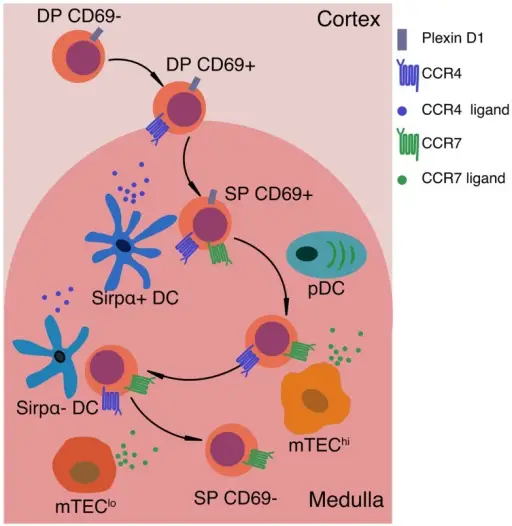
Signals that impact motility and localization of positively selected thymocytes. After positive selection, thymocytes up-regulate CD69 and the chemokine receptor CCR4. CCR4 ligands are expressed by medullary DCs, thus creating a chemotactic gradient that may promote medullary entry of post-positive selection thymocytes. PlexinD1 is expressed on DP and immature SP thymocytes, and may also promote medullary entry by inhibiting responses to cortical chemokines and releasing thymocytes from tight interactions with cTECs. As SP cells mature, they up-regulate CCR7, promoting chemotaxis toward the gradient of CCR7 ligands produced by mTEClo (CD80loMHC-IIlo) and mTEChi (CD80+MHC-IIhi) cells. CCR7 signaling is critical for maintaining SP thymocytes within the medulla. In the absence of CCR7, SP cells do not undergo efficient negative selection against TRAs. Expression of CCR7 and CCR4 on SP thymocytes may also promote chemokinesis, or rapid motility of SP thymocytes, as well as efficient interactions with the two main subsets of medullary APCs, mTEChi cells and DCs, respectively. Thus, chemokine-guided migration likely impacts multiple aspects of SP motility and cellular interactions that are required to ensure SP thymocytes efficiently scan numerous medullary APCs to encounter the full array of self-antigens that induce central tolerance. The Contribution of Chemokines and Migration to the Induction of Central Tolerance in the Thymus.
Hu Z, Lancaster JN, Ehrlich LI - Frontiers in immunology (2015). Not Altered. CC.