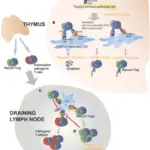
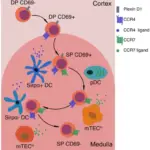
The role of suppression in immunologic tolerance is to reduce T cell reactivity and to maintain self-tolerance.
What is the Role of Suppression in Immunologic Tolerance?
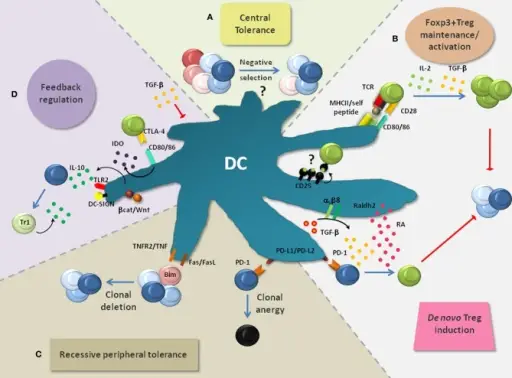
(A) DCs are implicated in the negative selection of self-reactive T cells and thus in central tolerance, although this is still a subject of intense research. (B) DCs are critically involved in the de novo generation, homeostasis, and activation of Foxp3+ Tregs which play a non-redundant role in the suppression of lethal autoimmunity. Foxp3+ Treg activation by DC-associated MHC-class-II: peptide complexes and CD80/CD86 is crucial to activate the immunosuppressive properties of Foxp3+ Tregs and to induce their expansion. Additionally, IL-2 and TGF-β, which can be produced by DCs, are critically involved in the maintenance of Treg function and proliferation. DC-derived TGF-β can also act on Foxp3− T cells to convert them into Foxp3+ Tregs. This process is enhanced by retinoic acid (RA) which is generated in DCs from retinaldehyde by the enzyme Raldh2. PD-1 ligands (PD-L1 and PD-L2) were also implied in Foxp3+ Treg induction. (C) Recessive peripheral tolerance is established in a T cell-intrinsic manner following instructive encounters with DCs. Antigenic activation of Foxp3− T cells can result in their functional inactivation (clonal anergy). This partly depends on the triggering of PD-1 on T cells via its ligands PD-L1 and PD-L2. Similarly, signaling via CTLA-4 can induce clonal anergy (not depicted). Another outcome of recessive tolerance can be clonal T cell deletion which depends on interactions via TNF/TNFR2, FasL/Fas, or induction of the proapoptotic factor Bim. (D) Multiple mechanisms can result in the feedback inhibition of DCs. Foxp3+ Tregs bind the co-stimulatory molecules CD80/CD86 on DCs via CTLA-4. This interaction leads to the production of indolamine-2,3-dioxygenase (IDO) and in the suppression of DC maturation (not depicted). IDO causes the apoptosis of conventional T cells and activates Foxp3+ Tregs. TGF-β produced and activated by DCs can directly inhibit DC functions. Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity: Mayer CT, Berod L, Sparwasser T - Frontiers in immunology (2012). Not altered. CC.