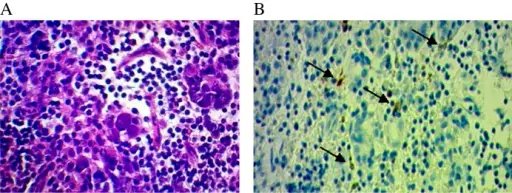
Pituitary gland pathology is the gross and microscopic study of the pituitary gland, it’s structural and functional changes in the presence of an illness with clinical manifestations such as hyperpituitarism, hypopituitarism, and local mass effects.
What are the Cells of the Pituitary Gland?
The anterior pituitary gland consists of acidophils, basophils, and chromophobe cell types.
Acidophils of the pituitary glands is a histological feature of the anterior pituitary, array of cells containing eosinophilic cytoplasm.
Basophils of the pituitary glands a histological feature of the anterior pituitary, array of cells containing basophil cytoplasm.
Chromophobes of the pituitary gland a histological feature of the anterior pituitary, array of cells containing poorly staining cytoplasm.
The six specific types of cells of the anterior pituitary gland include:
- Corticotrophs
- Gonadotrophs
- Lactotrophs
- Mammosomatotrophs
- Somatotrophs
- Thyrotrophs
| Type of Cell | Hormone Produced | The function of the Hormone |
| Corticotrophs | Adrenocorticotropic hormone (ACTH), Pro-opiomelanocortin (POMC), | Regulates the secretion of glucocorticoids by the adrenal cortex |
| Gonadotrophs | Follicle Stimulating Hormone (FSH), and luteinizing hormone (LH) | In men, FSH stimulates spermatogenesisand LH regulates Leydig cell function.In women, FSH is involved in the regulation of follicle growth,while LH is related to ovulation and development of the corpus luteum |
| Lactotrophs | Prolactin | This stimulates the breast during lactation |
| Mammosomatotrophs | Prolactin | This stimulates the breast during lactation |
| Somatotrophs | Growth Hormone (GH) | Direct effects, the stimulation of protein synthesis in liver and muscle and lipolysis of fat stores. Indirect effects, skeletal growth mediated by insulin-like growth factor I (IGF-I), which also exerts negative feedback on GH release from the pituitary |
| Thyrotrophs | Thyroid-Stimulating Hormone (TSH). | Stimulates the thyroidfollicular cells. |
What is Hyperpituitarism?
Hyperpituitarism is a clinical manifestation of the pathology of the pituitary gland characterized by spare secretion of trophic hormones, hyperfunction of the anterior pituitary gland.
What are the Effects of Local Mass Effects on the Pituitary Gland?
The effects of local mass effect on the pituitary gland are a feature associated with mass increases of the pituitary gland, visual field abnormalities caused by compression on optic nerves, and chiasm by expanding the pituitary gland. A pituitary adenoma may result in elevated Intracranial Pressure symptoms such as nausea, vomiting, and headache.
What is a Pituitary Adenoma?
A pituitary adenoma is a lesion to the anterior pituitary gland, encompassing around 10% of intracranial neoplasms in neurosurgical practice.
The different types of adenomas include:
| Pituitary Adenoma | Features |
| Corticotroph Adenomas | POMC Derived Peptides, ATCH. Cushing Syndrome, Nelson Syndrome. |
| Gonadotroph Adenomas | Follicle Stimulating Hormone. Luteinizing Hormone. Hypogonadism, Hypopituitarism |
| Lactotroph Adenomas | Prolactin. Amenorrhea in women, Infertility, Sexual dysfunction. |
| Mammosomatotroph Adenomas | Growth Hormone, Prolactin. Features of Growth Hormone and Prolactin Excess; Acromegaly in Adults, Gigantism in Children, Amenorrhea in women, Infertility, Sexual dysfunction. |
| Somatotroph Adenomas | Growth Hormone. Acromegaly in Adults, Gigantism in Children. |
| Thyrotroph Adenomas | Thyroid Stimulating Hormone. Hyperthyroidism. |
Pituitary adenomas are classified into different subtypes that include the following:
- Corticotroph adenomas
- Gonadotroph adenomas
- Lactotroph adenomas
- Mammosomatotroph adenomas
- Somatotroph adenomas
- Thyrotroph adenomas
What are Corticotroph adenomas?
Corticotroph adenomas are mostly basophilic and infrequently chromophobic, trivial microadenomas during diagnosis.
Associated syndromes include:
- Cushing syndrome
- Nelson syndrome
What is the Pathology of Corticotroph Adenomas?
The pathology of corticotroph adenomas is:
-Etiology: The cause of corticotroph adenomas is the amalgamation of molecular genetic changes and hormonal stimulation acting as a promoter.
-Genes involved: GNAS1 gene.
-Pathogenesis: The sequence of events that lead to corticotroph adenomas is as follows. The mutation and activation of Gsα result in the generation of cyclic adenosine monophosphate (cAMP), a potent mitogenic stimulus for variety of endocrine cell types, hence endorsing hormone synthesis and secretion and cellular proliferation.
-Morphology: The morphology associated with corticotroph adenomas shows that it may range in size from small termed microadenoma to large adenomas, spherical, soft, and encapsulated.
-Histology: The histology associated with corticotroph adenomas shows densely granulated basophils and sometimes sparsely granulated chromophobes.
How does Corticotroph Adenomas Present?
Patients with corticotroph adenomas are typically more frequent in females 3.5:1 female-to-male ratio presents at an age range of 30 and 40 years. The symptoms, features, and clinical findings associated with corticotroph adenomas include the symptoms of Cushing syndrome which include weight gain, muscle weakness, bone prone to fractures, tiredness, and mood disorders.
How are Corticotroph Adenomas Diagnosed?
Corticotroph adenomas are diagnosed through laboratory studies imaging and biopsy.
How are Corticotroph Adenomas Treated?
Corticotroph adenomas are treated with surgery, stereotactic radiosurgery, and adjuvant therapy.
What is the Prognosis of Corticotroph Adenomas?
The prognosis of corticotroph adenomas is poor as they tend to recur despite surgical intervention.
What are Gonadotroph adenomas?
Gonadotroph adenomas are type of adenomas that secrete the gonadotropins Follicle Stimulating hormone (FSH) and latinizing hormone. (LH).
Associated syndromes include:
- Hypogonadism
What is the Pathology of Gonadotroph Adenomas?
The pathology of gonadotroph adenomas is:
Etiology: The cause of gonadotroph adenomas is the amalgamation of molecular genetic changes and hormonal stimulation acting as a promoter.
-Genes involved: GNAS1 gene.
-Pathogenesis: The sequence of events that lead to gonadotroph adenomas is as follows. The mutation and activation of Gsα result in the generation of cyclic adenosine monophosphate (cAMP), a potent mitogenic stimulus for variety of endocrine cell types, hence endorsing hormone synthesis and secretion and cellular proliferation.
-Morphology: The morphology associated with gonadotroph adenomas shows that it may range in size from small termed microadenoma to large adenomas, spherical, soft, and encapsulated.
-Histology: The histology associated with gonadotroph adenomas shows chromophobic cells.
How does Gonadotroph adenomas Present?
Patients with gonadotroph adenomas typically common in more women than men present at age range of 30 to 45 years. The symptoms, features, and clinical findings associated with gonadotroph adenomas include diplopia, headaches, or pituitary apoplexy, reduced energy and libido in men.
How is Gonadotroph Adenomas Diagnosed?
Gonadotroph adenomas is diagnosed through laboratory studies imaging and biopsy.
How is Gonadotroph Adenomas Treated?
Gonadotroph adenomas is treated resection of the tumor.
What is the Prognosis of Gonadotroph Adenomas?
The prognosis of gonadotroph adenomas is fair as resection correct visual impairment diminishes endocrine dysfunction, resolve headaches.
What are Lactotroph Adenomas?
Lactotroph adenomas are frequent type of hyperfunctioning pituitary adenoma, responsible for 30% of clinically known pituitary adenomas.
Associated syndromes include:
- Galactorrhea
- Infertility
- Sexual dysfunction
What is the Pathology of Lactotroph Adenomas?
The pathology of lactotroph adenomas is:
– Etiology: The cause of gonadotroph adenomas is the amalgamation of molecular genetic changes and hormonal stimulation acting as a promoter.
-Genes involved: Pituitary tumor transforming gene (PTTG), GNAS1 gene, MicroRNA-7 (miR-7).
-Pathogenesis: The sequence of events that lead to gonadotroph adenomas is as follows. Arises from monoclonal growth of pituitary lactotrophs. The mutation and activation of Gsα result in the generation of cyclic adenosine monophosphate (cAMP), a potent mitogenic stimulus for variety of endocrine cell types, hence endorsing hormone synthesis and secretion and cellular proliferation.
-Morphology: The morphology associated with gonadotroph adenomas shows that it may range in size from small termed microadenoma to large adenomas, spherical, soft, and encapsulated.
-Histology: The histology associated with lactotroph adenomas shows a dimly acidophilic or chromophobic cells.
How does Lactotroph Adenomas Present?
Patients with lactotroph adenomas typically affect more women than men present at age range of 20 and 40 years. The symptoms, features, and clinical findings associated with lactotroph adenomas include amenorrhea, galactorrhea, loss of libido, and infertility.
How is Lactotroph Adenomas Diagnosed?
Lactotroph adenomas is diagnosed through laboratory studies; serum prolactin level, retrospectively biopsy.
How is Lactotroph Adenomas Treated?
Lactotroph adenomas is treated dopamine agonists therapy, surgical interventions.
What is the Prognosis of Lactotroph Adenomas?
The prognosis of lactotroph adenomas is good symptom managed medically.
What are Mammosomatotroph adenomas?
Mammosomatotroph adenomas are both GH and prolactin secreting tumors.
Associated syndromes include symptoms and syndromes of prolactin and growth hormone excess:
- Galactorrhea
- Infertility
- Sexual dysfunction
- Gigantism
- Acromegaly
What is the Pathology of Mammosomatotroph Adenomas?
The pathology of mammosomatotroph adenomas is:
-Etiology: The cause of mammosomatotroph adenomas is consolidation of molecular genetic changes and hormonal stimulation acting as a promoter.
-Genes involved: gene PRKAR1A, Menin (11q13).
-Pathogenesis: The sequence of events that lead to mammosomatotroph adenomas. Arises from growth of pituitary lactotrophs and somatotroph cell. The mutation and activation of Gsα result in the generation of cyclic adenosine monophosphate (cAMP), a potent mitogenic stimulus for variety of endocrine cell types, hence endorsing hormone synthesis and secretion and cellular proliferation.
-Morphology: The morphology associated with mammosomatotroph adenomas shows quite large adenoma.
-Histology: The histology associated with mammosomatotroph adenomas shows acidophilic on H & E staining.
How does Mammosomatotroph Adenomas Present?
Patients with mammosomatotroph adenomas typically more women than male present at age range of 20 to 45 years. The symptoms, features, and clinical findings associated with mammosomatotroph adenomas include acromegaly and hyperprolactinemia.
How is Mammosomatotroph Adenomas Diagnosed?
Mammosomatotroph adenomas is diagnosed through laboratory studies complex IHC study. Imaging studies such as high-resolution CT scan and/or MRI suggest an incidental lesion.
How is Mammosomatotroph Adenomas Treated?
Mammosomatotroph adenomas is treated dopamine antagonist, GH receptor antagonist and somatostatin receptor ligands, Surgical interventions.
What is the Prognosis of Mammosomatotroph Adenomas?
The prognosis of mammosomatotroph adenomas is poor, tend to be more aggressive than pure adenomas.
What are Somatotroph adenomas?
Somatotroph adenomas are second most common type of functioning pituitary adenoma.
Associated syndromes include:
- Gigantism
- Acromegaly
What is the Pathology of Somatotroph adenomas?
The pathology of somatotroph adenomas is:
-Etiology: The cause of somatotroph adenomas is genetic mutation and hormonal stimulation.
-Genes involved: Unknown.
-Pathogenesis: The sequence of events that lead to somatotroph adenomas. Arises from monoclonal growth of pituitary somatotroph cells. The mutation and activation of Gsα result in the generation of cyclic adenosine monophosphate (cAMP), a potent mitogenic stimulus for variety of endocrine cell types, hence endorsing hormone synthesis and secretion and cellular proliferation.
-Morphology: The morphology associated with somatotroph adenomas shows large adenoma.
-Histology: The histology associated with somatotroph adenomas shows densely granulated and sparsely granulated.
How does Somatotroph Adenomas Present?
Patients with somatotroph adenomas typically affect more female than male present at age range of 40 to 50 years old. The symptoms, features, and clinical findings associated with somatotroph adenomas include headaches and visual field flaws, increased body size with disproportionately long arms and legs, and hyperprolactinemia symptoms.
How are Somatotroph Adenomas Diagnosed?
Somatotroph adenomas is diagnosed through IHC study. Imaging studies; high-resolution CT scan and/or MRI suggest an incidental lesion.
How are Somatotroph Adenomas Treated?
Somatotroph adenomas is treated through surgical interventions, somatostatin analog therapy, and possibly radiation therapy.
What is the Prognosis of Somatotroph Adenomas?
The prognosis of somatotroph adenomas is poor as the condition is one of the silent adenomas dragonized when tumor is large.
What are Thyrotroph adenomas?
Thyrotroph adenomas are TSH-producing adenomas, rare, representing roughly 1% of all pituitary adenomas.
Associated syndromes include:
- Hyperthyroidism
What is the Pathology of Thyrotroph adenomas?
The pathology of thyrotroph adenomas is:
-Etiology: The cause of thyrotroph adenomas is hyperthyroidism, genetic changes
-Genes involved: Multiple endocrine neoplasia, type I (MEN1)
-Pathogenesis: The sequence of events that lead to thyrotroph adenomas is growth of pituitary thyrotroph cells. The mutation and activation of Gsα result in the generation of cyclic adenosine monophosphate (cAMP), a potent mitogenic stimulus for variety of endocrine cell types, hence endorsing hormone synthesis and secretion and cellular proliferation.
-Morphology: The morphology associated with thyrotroph adenomas shows its macroadenoma.
-Histology: The histology associated with thyrotroph adenomas shows it is chromophobic, with angular/ irregular cells.
How does Thyrotroph Adenomas Present?
Patients with thyrotroph adenomas typically has equal frequency male and female present at age range of 8 to 84 years old. The symptoms, features, and clinical findings associated with thyrotroph adenomas include hyperthyroidism signs and symptoms, visual field blemishes, headache.
How is Thyrotroph Adenomas Diagnosed?
Thyrotroph adenomas is diagnosed through laboratory studies, imaging, and biopsy.
How is Thyrotroph Adenomas Treated?
Thyrotroph adenomas is treated through surgical resection, pituitary radiotherapy, dopamine agonists, and somatostatin analogs.
What is the Prognosis of Thyrotroph Adenomas?
The prognosis of thyrotroph adenomas is good. Its recurrence does not seem to be frequent.
What are syndromes associated with pituitary gland pathology?
Syndromes associated with pituitary gland pathology are clinical manifestation associated with abnormality of an otherwise normal pituitary gland.
Syndromes associated with pituitary gland pathology include:
- Cushing syndrome
- Nelson syndrome
- Hypogonadism
- Galactorrhea
- Infertility
- Sexual dysfunction
- Gigantism
- Acromegaly
- Hyperthyroidism
What is Cushing’s syndrome?
Cushing syndrome is disease caused by chronic acquaintance to excessive circulating levels of glucocorticoids.
What is the Pathology of Cushing’s Syndrome?
The pathology of Cushing’s syndrome is:
-Etiology: The cause of Cushing’s syndrome is categorized into 2, ACTH-independent and ACTH-dependent.
-Genes involved: Unknown.
-Pathogenesis: The sequence of events that lead to Cushing’s syndrome is as follows, the loss 24-hour rhythm, combine with alteration feedback mechanism of the HPA axis, leads to continuing exposure to the undue circulating cortisol levels. This promotes to the quantifiable endogenic Cushing’s syndrome state.
-Morphology: Mass lesion.
-Histology: The histology associated with Cushing’s syndrome shows evidence of acinar expansion.
How does Cushing’s Syndrome Present?
Patients with Cushing’s syndrome typically more in females than males present at age range of 30 to 40 years old. The symptoms, features, and clinical findings associated with Cushing’s syndrome include growth failure, pubertal delay/arrest, visual disturbance obesity, and premature pubarche.
How is Cushing’s Syndrome Diagnosed?
Cushing’s syndrome is diagnosed through laboratory studies, dexamethasone suppression test urinary free cortisol (UFC) assay, steroids test, and corticotropin measurements.
How is Cushing’s Syndrome Treated?
Cushing’s syndrome is treated through treating the cause, medical steroid replacement therapy, radiation therapy, and surgery by transsphenoidal approach.
What is the Prognosis of Cushing’s Syndrome?
The prognosis of Cushing’s syndrome is poor linked to substantial morbidity due to multiple adverse effects.
What is Nelson syndrome?
Nelson syndrome is a hypothetically life-threatening ailment describing a range of signs associated with bilateral resection of the adrenal gland.
What is the Pathology of Nelson Syndrome?
The pathology of Nelson syndrome is:
-Etiology: The cause of Nelson syndrome is bilateral adrenalectomy, hypopituitarism.
-Genes involved: Unknown.
-Pathogenesis: The sequence of events that lead to Nelson syndrome; following bilateral adrenalectomy, cortisol absence, hypothalamus increase CRH production. Corticotropic cells in the pituitary gland respond to CRH by secretion of ACTH in huge quantities owing to feedback inhibition absence. The upsurge in CRH yields an effect on the corticotropic cells, which hypertrophy creates new growth or increases the original tumor. Surges in proopiomelanocortin production results from CRH elevation.
-Morphology: The morphology associated with Nelson syndrome shows the absence of bilateral adrenal, tumors are inclined to grow bigger.
-Histology: The histology associated with Nelson syndrome shows basophilic stain, episodic acidic-Schiff positivity.
How does Nelson Syndrome Present?
Patients with Nelson syndrome are typically females that present at the age range of up to 24 years old following initial bilateral adrenalectomy. The symptoms, features, and clinical findings associated with Nelson syndrome include hyperpigmentation, visual loss, bitemporal hemianopia, headache, fatigue, and weakness.
How is Nelson Syndrome Diagnosed?
Nelson syndrome is diagnosed through laboratory studies which include ACTH fasting plasma levels and brain MRI indicate a pituitary tumor.
How is Nelson Syndrome Treated?
Nelson syndrome is treated through surgical resection of the growth, stereotactic radiosurgery, fractionated radiotherapy, hormonal replacement therapy. Medical care, somatostatin-analogs, dopamine agonists, temozolomide, and sodium valproate.
What is the Prognosis of Nelson Syndrome?
The prognosis of Nelson syndrome is good if surgically treated.
What is Hypogonadism?
Hypogonadism is a syndrome in which the testis or the ovaries produces little or no hormones.
What is the Pathology of Hypogonadism?
The pathology of hypogonadism is:
-Etiology: The cause of hypogonadism is CNS disorders, genetic causes, congenital disorders, acquired disorders (hyperprolactinemia, Cushing syndrome) and Klinefelter syndrome.
-Genes involved: KISS and GNRH1, TAC3 and TACR3.
-Pathogenesis: The sequence of events that lead to hypogonadism, result from the incapability of the hypothalamic LHRH pulse creator and/or incapability to pituitary responding with LH and FSH secretion. Interruption of the hypothalamic-pituitary-gonadal axis. Gonad does not produce sex steroid adequate to overpower the secretion of LH and FSH.
-Morphology: Decreased size of gonads.
-Histology: NA.
How does Hypogonadism Present?
Patients with hypogonadism are typically common in males than females present at the age range of 15 to 45 years. The symptoms, features, and clinical findings associated with hypogonadism include libido and sexual dysfunction, hypospadias, virilization, micropenis among others.
How is Hypogonadism Diagnosed?
Hypogonadism is diagnosed through laboratory studies,
FSH and LH levels test, TFTs, prolactin levels, semen analysis, ACTH stimulation test. MRI of the brain.
How is Hypogonadism Treated?
Hypogonadism is treated through hormonal replacement.
What is the Prognosis of hypogonadism?
The prognosis of hypogonadism is good with no surge in mortality rate.
What is Galactorrhea?
Galactorrhea is a condition characterized by breast milk production unrelated to lactation or pregnancy in women.
What is the Pathology of Galactorrhea?
The pathology of galactorrhea is:
-Etiology: The cause of galactorrhea is hypothalamic-pituitary causes- prolactinomas, pituitary infiltrative disorders. Non-hypothalamic-pituitary etiology- hypothyroidism, medications (risperidone), renal failure, chest wall lesions, idiopathic.
-Genes involved: Unknown.
-Pathogenesis: The sequence of events that lead to galactorrhea. Vasoactive intestinal polypeptide and TRH stimulate prolactin production. Nipple stimulus, chest lesions, renal failure, and some medications may lead to hyperprolactinemia. Estrogens inhibition to hypothalamic dopamine also causes hyperprolactinemia.
-Morphology: Enlarged glandular tissue.
-Histology: Enlarged glands.
How does Galactorrhea Present?
Patients with galactorrhea are typically common in females present at the age range of 20 to 40 years. The symptoms, features, and clinical findings associated with galactorrhea include menstrual abnormalities, reduced libido, erectile dysfunction, and infertility. Green or white discharge from the breast.
How is Galactorrhea Diagnosed?
Galactorrhea is diagnosed through laboratory studies; prolactin level test, pituitary hormones assessment, and MRI of the pituitary gland.
How is Galactorrhea Treated?
Galactorrhea is treated through medical treatment such as dopamine agonists like bromocriptine.
What is the Prognosis of Galactorrhea?
The prognosis of galactorrhea is good has reduced the incidence of morbidity.
What is Infertility Related to Pituitary Gland Disorder?
Infertility related to pituitary gland disorder is linked to altered to the hypothalamic-pituitary-gonadal axis, and gonadotroph cell adenomas to the pituitary gland that causes instability of the axis.
What is the Pathology of Infertility Related to Pituitary Gland Disorder?
The pathology of infertility related to Pituitary gland disorder is:
-Etiology: The cause of infertility related to pituitary gland disorder is a tumor of the pituitary gland, hypopituitarism, hypogonadism
-Genes involved: Unknown.
-Pathogenesis: The sequence of events that lead to infertility related to pituitary gland disorder result from incapability of the hypothalamic LHRH pulse creator and/or incapability to pituitary responding with LH and FSH secretion. Interruption of the hypothalamic-pituitary-gonadal axis. Gonad does not produce sex steroids adequate to overpower the secretion of LH and FSH.
-Morphology: Infertile.
-Histology: NA.
How does Infertility relate to Pituitary Gland Disorder Present?
Patients with Infertility related to pituitary gland disorder typically affect both males and females present at the age range of 20 to 45 years. The symptoms, features, and clinical findings associated with infertility related to pituitary gland disorder include, gynecomastia, erectile dysfunction, and loss of libido.
How is Infertility related to Pituitary Gland Disorder Diagnosed?
Infertility related to pituitary gland disorder is diagnosed through laboratory studies such as FSH levels, LH levels test, TFTs, prolactin levels, semen analysis, ACTH stimulation test, and MRI of the brain.
How is Infertility related to Pituitary Gland Disorder Treated?
Infertility related to pituitary gland disorder is treated through hormonal replacement, treating underlying cause.
What is the Prognosis of Infertility Related to Pituitary Gland Disorder?
The prognosis of infertility related to pituitary gland disorder is good most respond well to treatment.
What is Sexual Dysfunction Related to Pituitary Gland Disorder?
Sexual dysfunction related to pituitary gland disorder is sexual hyperfunction and/or hypofunction linked to the diseased pituitary gland in males and females
What is the Pathology of Sexual Dysfunction Related to Pituitary Gland Disorder?
The pathology of sexual dysfunction related to pituitary gland disorder is:
-Etiology: The cause of sexual dysfunction related to pituitary gland disorder is tumors to the pituitary gland, conditions such as hypopituitarism, medication such as dopamine agonist.
-Genes involved: Unknown.
-Pathogenesis: The sequence of events that lead to sexual dysfunction related to pituitary gland disorder same as that of hypopituitarism, resulting from incapability of the hypothalamic LHRH pulse creator and/or incapability to pituitary responding with LH and FSH secretion. Interruption of the hypothalamic-pituitary-gonadal axis. Gonad does not produce sex steroids adequate to overpower the secretion of LH and FSH.
-Morphology: Gonadal abnormalities.
-Histology: Decreased spermatogenesis. Decreased ovulation.
How does Sexual Dysfunction Related to Pituitary Gland Disorder Present?
Patients with sexual dysfunction related to pituitary gland disorder typically affect more females than males present at an age range of 20 to 45 years. The symptoms, features, and clinical findings associated with sexual dysfunction related to pituitary gland disorder.
How is Sexual Dysfunction related to Pituitary Gland Disorder Diagnosed?
Sexual dysfunction related to pituitary gland disorder is diagnosed through laboratory studies, such as FSH levels, LH levels, TFTs, prolactin levels, semen analysis, and ACTH stimulation test.
How is Sexual Dysfunction related to Pituitary Gland Disorder Treated?
Sexual dysfunction related to pituitary gland disorder is treated through supportive therapy, hormonal replacement therapy, tapering down dopamine agonists in the treatment of hypopituitarism.
What is the Prognosis of Sexual Dysfunction related to Pituitary Gland Disorder?
The prognosis of sexual dysfunction related to pituitary gland disorder is good.
What is Gigantism?
Gigantism is an abnormality in which while the epiphyseal growth plates are open at 25 years and below, an oddly high linear growth occurs owing to the extreme action of insulin-like growth factor I (IGF-I).
What is the Pathology of Gigantism?
The pathology of gigantism is:
-Etiology: The cause of gigantism is the pituitary gland adenoma, excessive growth hormone.
-Genes involved: GPR101, Familial isolated pituitary adenoma (FIPA), PRKAR1A, GNAS, MEN1, CDKN1B, McCune-Albright syndrome (MAS),
-Pathogenesis: The sequence of events that lead to gigantism is the result of primary GH surplus, augmented GHRH production, and undue secretion of IGF-binding protein. Elevated tissue levels of free IGF-I, arbitrate most, of the growth-related consequences in gigantism.
-Morphology: The morphology associated with gigantism shows Increased stature
-Histology: The histology associated with gigantism shows sparsely/ densely granulated, Somatotrope carcinoma, Mixed somatotrope-lactotroph adenoma, infiltration of glycosaminoglycans, edematous and myxoid, slightly increased number of fibroblasts, and thinning of the epidermis.
How does Gigantism Present?
Patients with gigantism typically have equal prevalence in males and females present at an age range of 25 years and below. The symptoms, features, and clinical findings associated with gigantism include Endocrinopathies, visual changes in tall stature, headaches, macrocephaly, coarse facial structures, hyperhidrosis, osteoarthritis, cardiovascular disease, and tumors.
How is Gigantism Diagnosed?
Gigantism is diagnosed through laboratory studies to detect excess growth hormones, oral glucose tests, IGF elevated. Imaging; MRI for pituitary adenomas, CT scan evaluate the adrenal, pancreatic, ovarian tumors producing GH/GHRH
How is Gigantism Treated?
Gigantism is treated through medical care; dopamine and somatostatin analog, radiation therapy. Transsphenoidal surgery for adenoma removal may be needed.
What is the Prognosis of Gigantism?
The prognosis of gigantism is because an insignificant number of people with the condition, rates of mortality and morbidity throughout childhood are unidentified.
What is Acromegaly?
Acromegaly is a chronic metabolic disorder in which there is much growth hormone and the body tissue gradually enlarges.
What is the Pathology of Acromegaly?
The pathology of Acromegaly is:
-Etiology: The cause of acromegaly is the pituitary gland adenoma, excessive growth hormone.
-Genes involved: GPR101, Familial isolated pituitary adenoma (FIPA), PRKAR1A, GNAS, MEN1, CDKN1B, McCune-Albright syndrome (MAS).
-Pathogenesis: The sequence of events that lead to acromegaly is the result of primary GH surplus, augmented GHRH production, and undue secretion of IGF-binding protein. Elevated tissue levels of free IGF-I, arbitrate most, of growth-related consequences in gigantism and acromegaly.
-Morphology: The morphology associated with acromegaly shows increased stature.
-Histology: The histology associated with acromegaly shows somatotrope carcinoma, Mixed somatotrope-lactotrope adenoma, infiltration of glycosaminoglycans, edematous and myxoid, slightly increased number of fibroblasts, and thinning of the epidermis.
How does Acromegaly Present?
Patients with acromegaly typically affect males and females in equal prevalence present at an age range of 15 to 45 years. The symptoms, features, and clinical findings associated with acromegaly include endocrinopathies, visual changes tall stature, headaches, macrocephaly, coarse facial structures, hyperhidrosis, osteoarthritis, cardiovascular disease, and tumors.
How is Acromegaly Diagnosed?
Acromegaly is diagnosed through laboratory studies to detect excess growth hormones, oral glucose test, IGF elevated. Imaging; MRI for pituitary adenomas, CT scan evaluate the adrenal, pancreatic, ovarian tumors producing GH/GHRH
How is Acromegaly Treated?
Acromegaly is treated through medical care; dopamine and somatostatin analog, radiation therapy. Transsphenoidal surgery for adenoma removal may be useful.
What is the Prognosis of Acromegaly?
The prognosis of Acromegaly is poor. Severe ailment, often lately diagnosed, its mortality, morbidity rates are high.
What is Hyperthyroidism?
Hyperthyroidism is a syndrome allied to excess thyroid hormone production and secretions.
What is the Pathology of Hyperthyroidism?
The pathology of hyperthyroidism is:
-Etiology: The cause of hyperthyroidism is an autoimmune disease Graves’ disease, multinodular goiter, and thyroxine-secreting tissue.
-Genes involved: TSH receptor gene (TSHR).
-Pathogenesis: The sequence of events that lead to hyperthyroidism, underlying autoimmune cause, there is the production of thyroid-stimulating immunoglobulins which bind to the TSH receptor, mimic the TSH effects. Autonomous ectopic tissue in multinodular goiter causes excess production of thyroid hormone, causing clinical thyrotoxicosis.
-Morphology: The morphology associated with hyperthyroidism shows diffuse and toxic nodular hyperplasia.
-Histology: The histology associated with hyperthyroidism shows lymphocytic penetration, diffuse follicular and papillary hyperplasia.
How does Hyperthyroidism Present?
Patients with hyperthyroidism typically more in females (2.7%) than males (0.23%) present at age range 20-40 years. The symptoms, features, and clinical findings associated with hyperthyroidism include anxiety, hyperactivity, increased perspiration, Nervousness, Palpitations, muscle weakness heat intolerance tachycardia, hand tremor.
How is Hyperthyroidism Diagnosed?
Hyperthyroidism is diagnosed through history and physical examination. Laboratory studies for thyroid function test indicates reduced TSH and increased T3 and T4, anti-thyroid peroxidase (anti-TPO) antibody test, autoantibody titers, scintigraphy, and radioisotope thyroid scan may be useful.
How is Hyperthyroidism Treated?
Hyperthyroidism is treated through symptoms relief, thionamide therapy, iodine radioactive therapy, and subtotal thyroidectomy.
What is the Prognosis of Hyperthyroidism?
The prognosis of hyperthyroidism is good disease is associated with definitive positive treatment.
What is hypopituitarism?
Hypopituitarism is a clinical condition characterized by a decrease in pituitary hormone secretion, resulting from disorders relating to the hypothalamus, pituitary gland, or surrounding structures.
Causes of hypopituitarism include:
- Apoplexy
- Empty sella syndrome
- Genetic defects
- Infections
- Inflammation
- Ischemic necrosis
- Mass lesions
- Radiation
- Rathke cleft cyst
- Surgery
- Trauma
- Tumors
How does Apoplexy cause hypopituitarism?
Apoplexy causes hypopituitarism by increased compression of the normal pituitary gland.
How does Empty Sella Syndrome cause hypopituitarism?
Empty sella syndrome causes hypopituitarism by the effect of arachnoid herniation into the sella turcica, flattening the pituitary gland against nearby bone.
How does Genetic Defects cause hypopituitarism?
Genetic defects causes hypopituitarism by initiating abnormal pituitary gland development resulting in insufficient functioning of the pituitary gland.
How do Infections cause hypopituitarism?
Infections may cause hypopituitarism by their spread to the brain, strategically placed tuberculoma, vasculitis, or exudates around the sellar region, interfering with the normal function of the pituitary gland.
How does Inflammation Cause Hypopituitarism?
Inflammation causes hypopituitarism by causing swelling and sometimes loss of function of the pituitary gland.
How does Ischemic Necrosis Cause Hypopituitarism?
Ischemic necrosis causes hypopituitarism by the destruction of pituitary tissues leading to its loss of functionality.
How do Mass Lesions Cause Hypopituitarism?
Mass Lesions causes hypopituitarism by compression of the gland, interfering with hypothalamus-thyroid- pituitary axis.
How does Radiation Cause Hypopituitarism?
Radiation causes hypopituitarism by causing irreversible and progressive injuries of the hypothalamic-pituitary area.
How does Rathke Cleft Cyst Cause Hypopituitarism?
Rathke cleft cyst causes hypopituitarism by causing compression of the pituitary interfering with its functions.
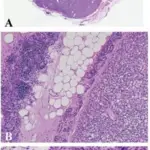
What is a Rathke Cleft Cyst?
A Rathke cleft cyst is a benign fluid-filled lesion that develops between the structure of the anterior pituitary gland at the base of the brain hypothesized to instigate from fragments of Rathke pouch.
What is the Pathology of Rathke Cleft Cyst?
The pathology of rathke cleft cyst is:
-Etiology: The cause of rathke cleft cyst is the failure of rathke cleft to regress.
-Genes involved: FOXA1, and FOXJ1.
-Pathogenesis: The sequence of events that lead to rathke cleft cyst, resulting from true fragments of the embryologic Rathke pouch, the pouch residual lumen reduced to a thin Rathke cleft. This cleft is supposed to regress. Its persistence leads to rathke cleft cyst
-Morphology: The morphology associated with rathke cleft cyst shows thin presenting with different colors, (blue, yellow, gray, white, pink, red, or green). Varying in size 10 and 20mm, gelatinous yellow fluid.
-Histology: The histology associated with rathke cleft cyst shows vascularized stroma of connective tissue, mucous secreting ciliated, and non-ciliated epithelial cells.
How does Rathke Cleft Cyst Present?
Patients with rathke cleft cyst typically have a 2:1 female to male ratio present at age range 30-50 years of age. The symptoms, features, and clinical findings associated with rathke cleft cyst include chiasmatic syndrome, vision changes, headaches, Nausea, fatigue, rare epilepsy, symptoms of hypopituitarism, growth retardation. low libido, and impotence.
How is Rathke Cleft Cyst Diagnosed?
Rathke cleft cyst is diagnosed through radiography, plain skull indicates abnormal configured sellar, CT scan and brain MRI.
How is Rathke Cleft Cyst Treated?
Rathke cleft cyst is treated through transsphenoidal surgery.
What is the Prognosis of Rathke Cleft Cyst?
The prognosis of a rathke cleft cyst is good.
How does Surgery Cause Hypopituitarism?
Surgery causes hypopituitarism by removal of the tumor of the pituitary gland interfering with the normal function of the pituitary gland.
How does Trauma Cause Hypopituitarism?
Trauma may cause hypopituitarism by direct injury to the pituitary structures.
How do Tumors Cause Hypopituitarism?
Tumors may cause hypopituitarism by making the pituitary overproduce hormones, may compress the gland leading to little being produce causing hypopituitarism.
What are Posterior Pituitary Syndromes?
Posterior pituitary syndromes are medical conditions associated with defects in the production of ADH from the posterior pituitary.
Examples of posterior pituitary syndromes include:
- Central diabetes insipidus
- Syndrome of inappropriate ADH secretion
What is Central Diabetes Insipidus?
Central diabetes insipidus is a condition caused by ADH insufficiency branded by polyuria owing to the incapability of the kidney to reabsorb water properly from the urine.
What is Nephrogenic Diabetes Insipidus?
Nephrogenic diabetes insipidus is a condition caused by the unresponsiveness of renal tubular to circulating ADH characterized by polyuria owing to the incapability of the kidney to reabsorb water properly from the urine.
What is Syndrome of Inappropriate ADH secretion?
Syndrome of Inappropriate ADH secretion is a condition in which there is resorption of excessive amounts of free water results in hyponatremia, caused by excess ADH.
What are Hypothalamic Suprasellar Tumors?
Hypothalamic suprasellar tumors are neoplasms arising in the suprasellar region capable of inducing hyperfunction or hypofunction of the anterior pituitary, diabetes insipidus, or combination.
Examples of hypothalamic suprasellar tumors include:
- Gliomas
- Craniopharyngiomas
What are Gliomas?
Gliomas are tumor lesions mostly occurring in the brain stem within the pons, midbrain, and medulla, which may infiltrate past the brainstem.
What is the Pathology of Gliomas?
The pathology of gliomas is:
-Etiology: The cause of gliomas is genetic or environmental.
-Genes involved: PDGF-A, RB gene, the p16/CDKNZA gene.
-Pathogenesis: The sequence of events that lead to gliomas, not well known.
-Morphology: The morphology associated with gliomas shows differentiated (astrocytoma) or less differentiated (higher-grade), ranging from anaplastic astrocytoma to glioblastoma, range in size from a few centimeters to enormous lesions.
-Histology: The histology associated with gliomas shows noticeable hypercellularity, microvascular proliferation, nuclear atypia, and necrosis.
How does Gliomas Present?
Patients with gliomas typically have no gender prevalence present at an age range of 10 to 60 years. The symptoms, features, and clinical findings associated with gliomas include unsteady gait, dysarthria, dysphagia, drowsiness, headache, rare seizures, failure to thrive, long tract signs, and ataxia.
How are Gliomas Diagnosed?
Gliomas are diagnosed through laboratory studies including CSF examination.
How is Gliomas Treated?
Gliomas are treated through surgical resection, radiotherapy, or chemotherapy.
What is the Prognosis of Gliomas?
The prognosis of gliomas is good, with longer survival allied to the tectal and cervicomedullary gliomas. Poor prognosis allied to intrinsic pontine gliomas with a 5-year relative survival of 5%.
What are Craniopharyngiomas?
Craniopharyngiomas are rare intracranial dysontogenic tumors with benign histology and malignant behavior
What is the Pathology of Craniopharyngiomas?
The pathology of craniopharyngiomas is:
-Etiology: The cause of craniopharyngiomas is fragments of the Rathke cleft and craniopharyngeal duct and gene mutation and involvement.
-Genes involved: Beta-catenin gene, BRAF (V600E)
-Pathogenesis: The sequence of events that lead to craniopharyngiomas; arise from the proliferation of epithelial-squamous cells lining the pituitary stalk.
-Morphology: The morphology associated with craniopharyngiomas shows large cysts or multiple cysts with solid components, fibrous tissue necrotic debris, and calcifications.
-Histology: The histology associated with craniopharyngiomas shows the basal layer of trivial cells, darkly stained nuclei and slight cytoplasm, keratinized to flat plate-like squamous cells.
How does Craniopharyngiomas Present?
Patients with craniopharyngiomas typically has no gender prevalence, present at age range of 5 to 65 years The symptoms, features, and clinical findings associated with craniopharyngiomas include endocrine dysfunction, headache visual disturbances, cold intolerance, weight gain, excessive fatigue, adrenal failure symptoms diabetes insipidus and reduced sexual drive.
How are Craniopharyngiomas Diagnosed?
Craniopharyngiomas are diagnosed through laboratory studies, complete endocrine evaluation (serum and urine osmolality thyroid functioning tests, growth hormone levels).
How is Craniopharyngiomas Treated?
Craniopharyngiomas are treated through surgical resection, radiation therapy, or chemotherapy.
What is the Prognosis of Craniopharyngiomas?
The prognosis of craniopharyngiomas is good.